Table 4.
In Vitro Growth Inhibitory Effects of the SMART Compounds with different “A” Rings against the Proliferation of Melanoma (A 375, B16-F1) and Prostate Cancer Cells (DU145, PC-3, LNCaP, PPC-1).
| Compounds 8 | A Ring | IC50 ± SEM (nM) |
||||||
|---|---|---|---|---|---|---|---|---|
| B16-F1 | A375 | DU 145 | PC-3 | LNCaP | PPC-1 | |||
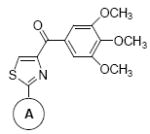 |
8f | Ph | 55 ± 5 | 28 ± 5 | 71 ± 4 | 21 ± 1 | 28 ± 4 | 43 ± 5 |
| 8k | 4-Methyl-Ph | 21 ± 10 | 11 ± 5 | 7 ± 1 | 5± 1 | 6 ± 1 | 6 ± 1 | |
| 8l | 2-Fluoro-Ph | 27 ± 11 | 30 ± 9 | 114 ± 3 | 82 ±9 | 53 ± 4 | 52 ± 3 | |
| 8m | 3-Fluoro-Ph | 287 ± 36 | 304 ± 25 | 35 ± 3 | 24 ± 2 | 11 ± 2 | 21 ± 1 | |
| 8n | 4-Fluoro-Ph | 43 ± 21 | 33 ± 14 | 12 ± 1 | 13 ± 1 | 6 ± 1 | 8 ± 1 | |
| 8o | 3, 4-Dimethoxy-Ph | 161 ± 29 | 34 ± 10 | 102 ± 2 | 69 ± 3 | 38 ± 6 | 56 ± 2 | |
| 8p | 4-Nitro-Ph | 56 ± 12 | 38 ± 9 | 95 ± 5 | 56 ± 1 | 39 ± 4 | 34 ±1 | |
| 8q | 4-Cyano-Ph | 53 ± 16 | 59 ± 24 | 52 ± 2 | 30 ± 7 | 15 ±4 | 19 ± 2 | |
| 8t | 4-Trifluoromethyl-Ph | 92 ± 16 | 23 ± 5 | 50 ± 5 | 58 ± 4 | 94 ± 1 | 76 ± 1 | |
| 8u | 4-Bromo-Ph | 32 ± 5 | 13 ± 2 | 21 ± 4 | 18 ± 3 | 44 ± 3 | 21 ± 5 | |
| 8v | 4-Ethyl-Ph | 70 ± 8 | 17± 2 | 31 ± 4 | 27 ± 4 | 60 ± 5 | 22 ± 3 | |
| 8x | 4-Pyridine | >100000 | >100000 | >20000 | >20000 | >20000 | >20000 | |
| 8y | 2-Pyrimidine | 2300 ± 860 | 4100 ± 740 | 2813 ± 92 | 2657 ± 40 | 2370 ± 85 | 1186 ± 22 | |
| 8z | 2-Thienyl | 38 ± 15 | 20 ± 7 | 22 ± 1 | 17 ± 2 | 9 ± 1 | 13 ± 1 | |
| 10 | Ha | >100000 | >100000 | >20000 | >20000 | >20000 | >20000 | |
Compound 10 has a proton at “A” ring position.
