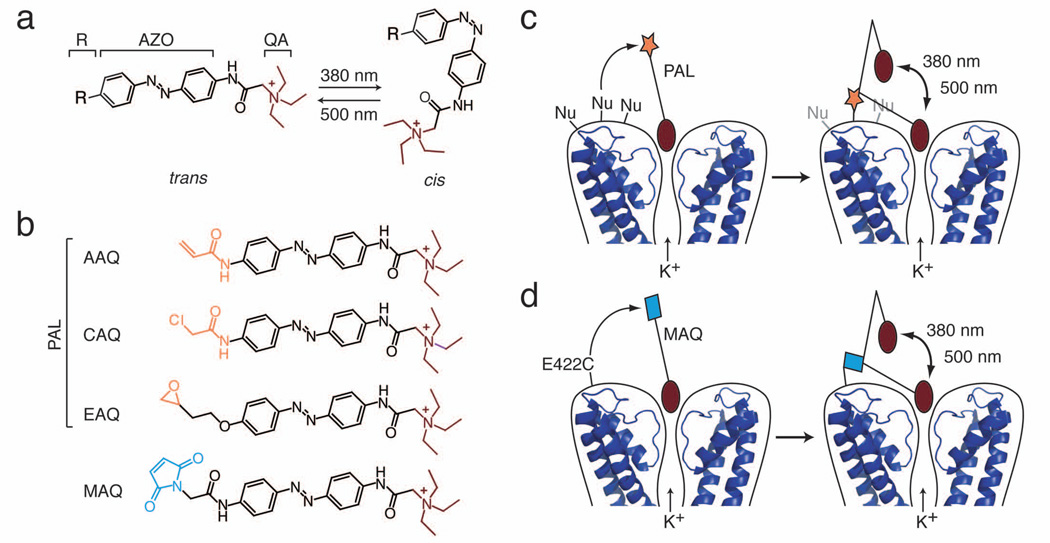
Figure 1. The PAL approach for imparting light sensitivity onto native ion channels.
(a) PAL molecules consist of a photoisomerizable azobenzene group (AZO) flanked by a quaternary ammonium (QA; burgundy) and a covalent attachment group (R). Exposure to 380 nm light isomerizes the AZO to its shorter cis form whereas exposure to 500 nm light favors the trans configuration.
(b) PAL molecules contain a promiscuous reactive group (orange): acrylamide (Acryl-AZO-QA or AAQ), chloroacetamide (CAQ), or epoxide (EAQ). In contrast, MAQ contains a maleimide (blue) designed to react with an engineered cysteine.
(c) After QA binds to the channel pore, PAL reacts via its promiscuous reactive group with an endogenous nucleophile (Nu) on native K+ channels. Once tethered, the photoswitch allows control of ionic current using light. In 500 nm light, the photoswitch is extended, blocking ion conduction. In 380 nm light, AZO isomerizes to its cis form, retracting the QA and allowing ion conduction. Molecular coordinates from KcsA (PDB ID: 2A9H) were drawn using MacPyMol (DeLano, W.L. The PyMOL Molecular Graphics System (2002); http://www.pymol.org).
(d) To generate the SPARK channel, the maleimide group of MAQ attaches to a cysteine (E422C) genetically engineered in a Shaker K+ channel.