Figure 3.
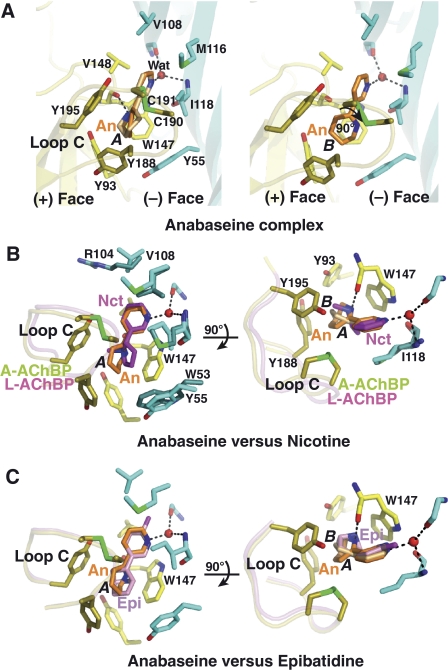
The anabaseine–AChBP complex: close-up view and structural comparisons. (A) The subunit interface is oriented with its apical side at top and its ‘membrane' side at bottom (same orientation as in Figure 2, column 2). The tip of loop C harbouring the Cys 190–Cys 191 disulfide is highlighted in green. The high affinity cyclic form of anabaseine, conformer A (left) and B (right), is bound between the disulfide above it and Trp 147 below it. Side chains and solvent molecules that interact specifically with bound anabaseine are shown. Critical hydrogen bond with the Trp 147 carbonyl is observed in conformer A. Superimposition of anabaseine bound to A-AChBP (conformers A and B) with (B) nicotine bound to L-AChBP (Celie et al, 2004) and (C) epibatidine bound to A-AChBP (Hansen et al, 2005), viewed in two orientations rotated by 90°. Retention of the closed conformation of loop C is evident.