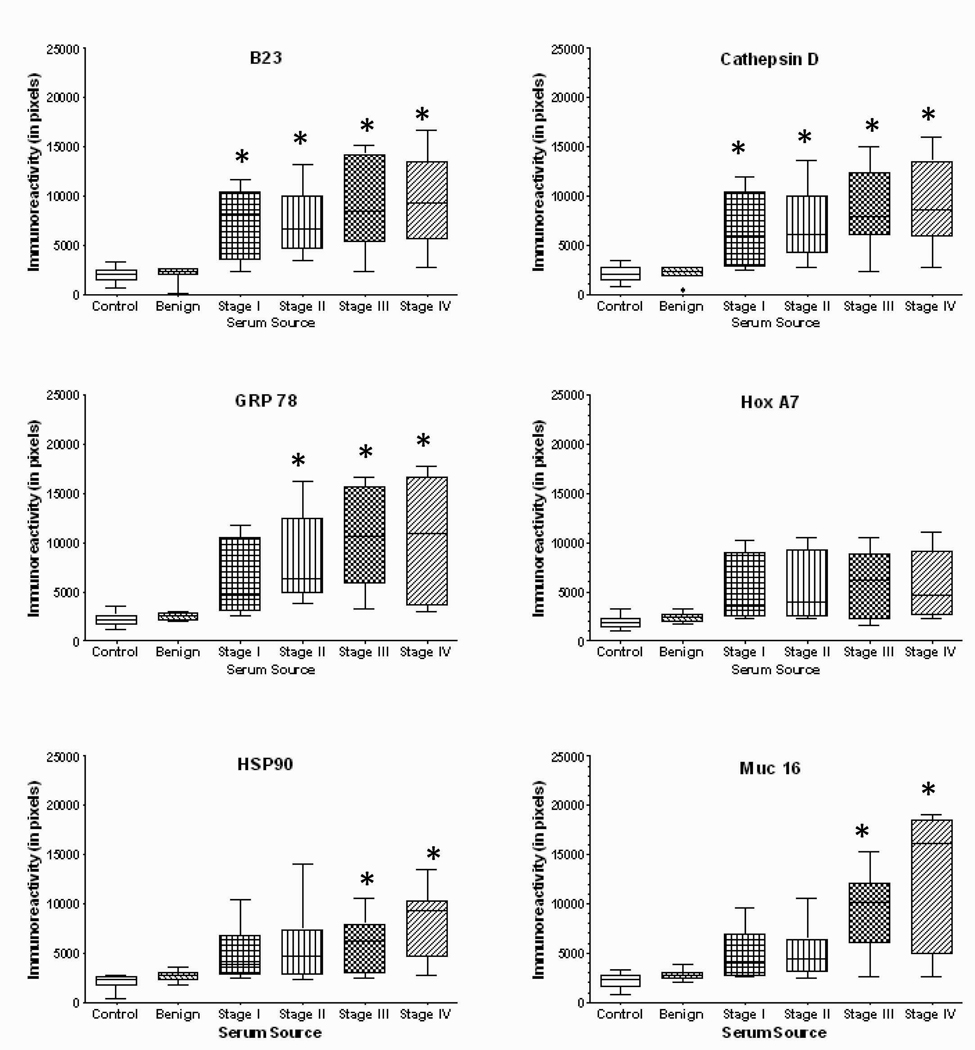
Figure 4.
Quantitation of dot immunoblots presenting the immunoreactivity of sera from normal controls (n=40), women with benign disease (n=40) and patients with various stages of ovarian cancer (Stage I, n=35; Stage II, n=25; Stage III, n=40; Stage IV, n=25) patients against nucleophosmin, cathepsin D, GRP78, HoxA7, HSP90, Muc16, NY-ESO-1, p53, PLAP, SSX common antigen, survivin, and TAG72. * denotes significantly different (p<0.05) than either control or benign.