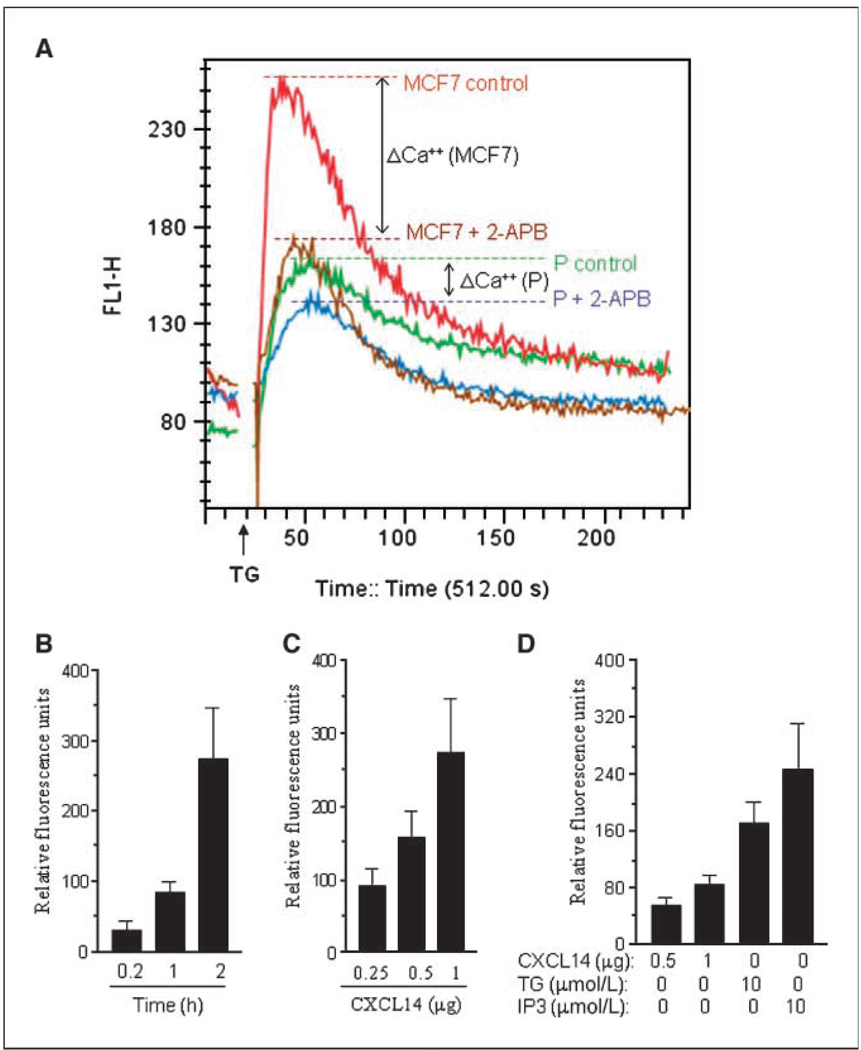
Figure 6.
CXCL14-induced Ca2+ release in microsomes and its inhibition by 2-APB in clone P in vitro. A, CXCL14 prevented the inhibition of IP3R by 2-APB in MCF7 subclones. Cells were loaded with 5 µmol/L Fluo-3 for 1h. MCF7 (red curve) and clone P (green curve) were treated with 2 µmol/L thapsigargin (TG) to deplete intracellular stores and activate capacitative calcium entry. MCF7 (brown curve) and clone P (blue curve) were treated with 100 µmol/L 2-APB for 10 min before adding 2 µmol/L thapsigargin. The results of a representative recording of the free Ca2+ measured by Fluo-3 fluorescence using flow cytometry as described in Materials and Methods are shown (n = 3). B, CXCL14 induced Ca2+ release from microsomes in a time-dependent manner. Microsomes were incubated with 1 µg CXCL14 protein, and at the indicated time, they were spun down and the supernatant containing Ca2+ was kept to further evaluate Ca2+ released. C, CXCL14 induced Ca2+ release from microsomes in a dose-dependent manner. Microsomes were incubated with up to 1 µg CXCL14 protein for 2 h and then were spun down and the supernatant was used to evaluate Ca2+ released. D, comparison of Ca2+ released from microsomes induced by IP3, thapsigargin, or CXCL14. Microsomes were incubated with 0.5 or 1 µg of CXCL14 protein, 10 µmol/L IP3, or 1 0µmol/L thapsigargin for 1 h and then spun down, and the supernatant was used to evaluate Ca2+ released. Columns, mean of at least three independent experiments; bars, SD.