Figure 5.
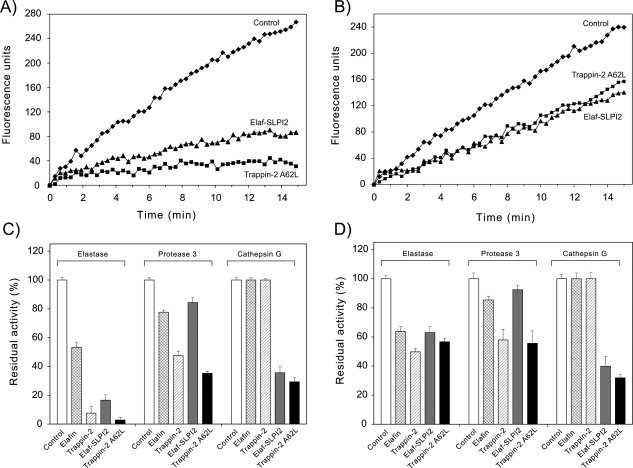
Inhibition of soluble NSPs by inhibitors cross-linked to fibronectin or elastin by transglutamination. Inhibitors were cross-linked to fibronectin or elastin in 96-well microplates essentially as described in (18). Inhibitors (10−6M) were incubated with tissue transglutaminase for 2 h at 37°C to form conjugated complexes with fibronectin (A,C) or elastin (B, D). The wells were washed thoroughly to remove unreacted products, and incubated with HNE (1 nM), Pr3 (2 nM), or CatG (2 nM) for 15 min at 37°C to allow protease-inhibitor complex formation. Residual enzyme activity was monitored using specific fluorogenic substrates. Representative inhibition curves (substrate hydrolysis expressed as fluorescence units vs time) are shown for the inhibition of HNE by Elaf-SLPI2 or trappin-2 A62L bound to fibronectin (A) or elastin (B). (C), (D), Graph show the residual enzyme activity for various protease-immobilized inhibitor pairs after cross-linking of the inhibitor to fibronectin (C) or elastin (D), calculated as the ratio of the rate of substrate hydrolysis in the presence of inhibitor to the rate of substrate hydrolysis without inhibitor (Control). Data are means ± SD for three separate experiments. The polyvalent inhibitors, Elaf-SLPI2 and trappin-2 A62L, inhibited all three NSPs when cross-linked to fibronectin or elastin, although Elaf-SLPI2 appeared to be less efficient against Pr3 than against HNE and CatG. As expected elafin and trappin-2 bound to fibronectin or elastin inhibited HNE and Pr3 but not CatG.
