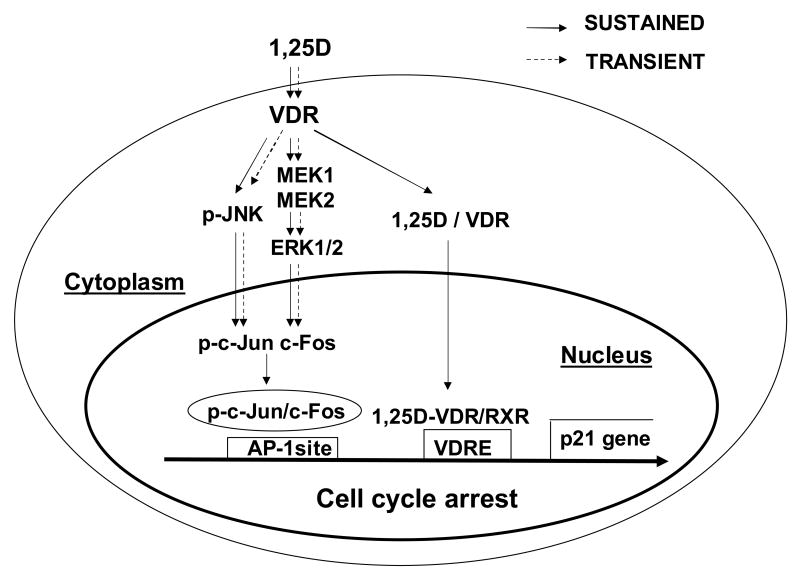
Fig. 6.
Proposed model for the integration of anti-proliferative genomic and nongenomic 1,25D pathways via MAPK cross-talk through a classic VDR in human osteosarcoma cells. Shortly after arrival at the cell surface, 1,25D hormone interacts with a VDR protein localized at the cytoplasm in close proximity to the plasma membrane that triggers nongenomic effects of the steroid. Cytoplasmic 1,25D/VDR complex rapidly (transient pathway) activates a JNK MAP kinase, on one hand, and upregulates c-Fos expression via a MEK1/MEK2/ERK pathway, on the other. Only under sustained presence of hormone, phosphorylated c-Jun, a downstream product of JNK activation, dimerizes with c-Fos at the cell nucleus, and the complex binds to an AP-1 site upstream the p21waf1 gene involved in the control of cell cycle progression. In addition, under continuous presence of hormone and according to traditional genomic pathways, 1,25D/VDR complex translocates to the cell nucleus and binds to a vitamin D response element (VDRE) upstream of the p21 gene. As a result of this genomic and nongenomic cross-talk, the cdk inhibitor p21 is expressed leading to cell cycle arrest and decreased cell proliferation in a time frame of days. As seen from our results, a classic VDR is required for nongenomic activation of MAPK signaling.