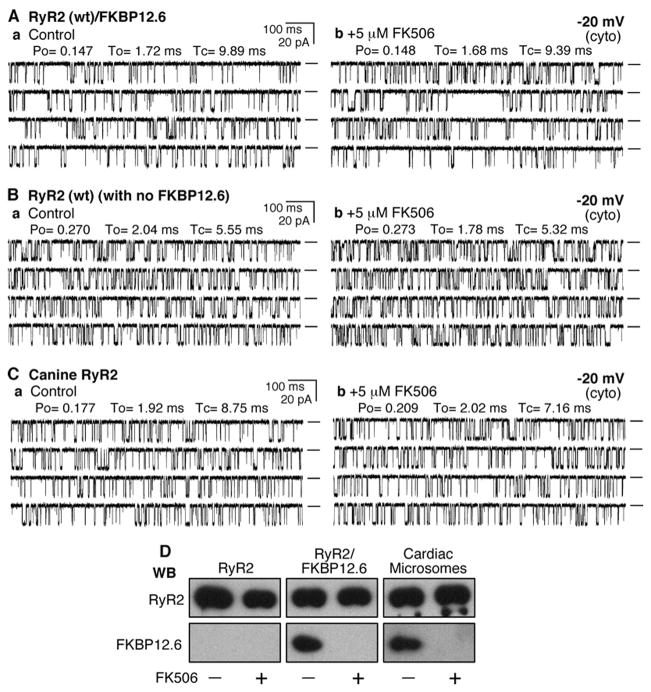
FIGURE 1. FKBP12.6-removal does not affect the conductance of single RyR2 channels.
Single channel activities of the recombinant RyR2 WT co-expressed with FKBP12.6 (A), recombinant RyR2 WT expressed alone (B), and the native canine RyR2 (C) were recorded in a symmetrical recording solution containing 250 mM KCl and 25 mM Hepes (pH 7.4) at a holding potential of −20 mV. EGTA was added to either the cis or trans chamber to determine the orientation of the incorporated channel. The side of the channel to which the addition of EGTA inhibited the activity of the incorporated channel presumably corresponds to the cytosolic face of the channel. Panel a shows control single channel activities in the presence of ~100 –200 nM cytosolic Ca2+and 45 nM luminal Ca2+. Single channel activities ~10 min after the addition of 5 μM FK506 are shown in panel b. A total of 7 RyR2/FKBP12.6, 10 RyR2 without FKBP12.6, and 8 canine RyR2 channels were tested, and none showed FK506-induced long-lived subconductance states. Openings are downward. Open probability (Po), arithmetic mean open time (To), and arithmetic mean closed time (Tc) are indicated at the top of the traces. A short line to the right of each current trace indicates the base line. D, Western blots (WB)of RyR2 and FKBP12.6 immunoprecipitated with an anti-RyR antibody (34c) from either HEK293 cell lysate expressing RyR2 or co-expressing RyR2 and FKBP12.6 or solubilized canine cardiac microsomes treated with or without 5 μM FK506.