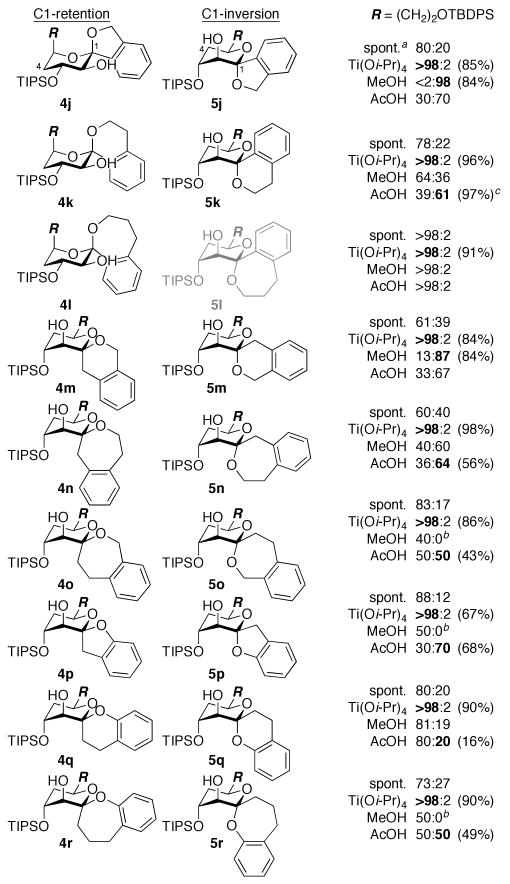
Figure 3.
Diastereomeric ratios of benzannulated spiroketals formed from d-erythro-glycal epoxides 3j–r. Isolated yields of 4 (Ti[Oi-Pr]4) and 5 (MeOH or AcOH) shown in parentheses. a Spontaneous spirocyclization (−78 °C → rt). b Remainder methyl glycoside 6. c Inseparable mixture of 4 and 5 (separable after desilylation).