Abstract
Ischemic events in humans are not evenly distributed across the day. To discriminate between temporal differences in the incidence of ischemia and susceptibility to ischemic events, we examined the outcome of global ischemia in a murine model at three time points during the day. Global cerebral ischemia in mice during the light phase impairs survival and exacerbates outcome compared to ischemia at other times of the day. Specifically, mice that underwent cardiac arrest during the light phase had greater numbers of degenerating neurons, greater microglial activation, and increased proinflammatory cytokine production in the ischemia-vulnerable hippocampus, as well as increased locomotor activity. Time-of-day differences were not altered by the melatonin receptor antagonist luzindole. Our results document that brain tissue displays endogenous fluctuations in susceptibility to ischemic damage and demonstrate that small differences in time of onset can significantly influence ischemic outcomes.
Keywords: Global ischemia, circadian rhythms, microglia, proinflammatory cytokines, cardiac arrest, heart disease
Introduction
Ischemic cardiovascular events including silent myocardial ischemia, acute myocardial infarction, sudden cardiac death, and stroke, exhibit marked time-of-day variation with an excess of occurrences in the early morning hours (Cohen et al., 1997; Muller et al., 1987; Rocco et al., 1987; Thompson et al., 1985; Willich et al., 1987). A confluence of physiological factors may contribute to this temporal variation in ischemic events, including daily maxima in blood pressure, blood coagulability, heart rate, vascular and sympathetic tone, and several neuroendocrine systems (Portaluppi and Lemmer, 2007). Although there is little doubt that temporal variation exists in the incidence of ischemic cardiovascular events, it remains unknown whether there is daily variation in susceptibility of the brain and other organ systems to ischemic damage.
Circadian organization of physiological processes has been demonstrated across phylogeny ranging from unicellular organisms to the highest order vertebrates (Ishiura et al., 1998; Reppert and Weaver, 2001). Among vertebrates, endogenous circadian rhythms can be detected in whole organisms, individual organs and tissues, and even single cells (Abe et al., 2002; Stokkan et al., 2001). Indeed, neurons exhibit daily rhythms in transcription, translation, synaptic activity, and neurotransmitter and neuropeptide secretion (Abe et al., 2002; Herzog, 2007). The overriding question motivating this study is do daily rhythms in physiology and behavior have potential consequences for susceptibility to disease states?
The central nervous system (CNS) is differentially susceptible to damage in cases of whole-body ischemia and CNS injury is often the proximate cause of death and long term disability from cardiac arrest (Becker et al., 1991; Roine et al., 1993); (Krause et al., 1986). Despite extensive research into the pathophysiology of cerebral ischemia, no broadly effective pharmacotherapy exists (Jastremski et al., 1989; Kofler et al., 2004; Roine et al., 1993). Therefore, the study of endogenous neuroprotective mechanisms that might provide insights into potential interventions is important (Perez-Pinzon, 2007). The experiments in this study were designed to test the hypothesis that CNS tissue exhibits daily fluctuations in susceptibility to ischemic damage and to begin to uncover the mechanisms that underlie temporal fluctuations in susceptibility. In order to address this question, a standardized ischemic challenge, cardiac arrest and cardiovascular resuscitation, (CA/CPR) was administered to healthy adult male mice at three different phases of the day [Middle of the light period, (Mid-Light) at the light-dark transition (Light→Dark), and in the middle of the dark period (Mid-Dark)]. To control for nonspecific effects of the surgical procedure, we also conducted the cardiac arrest and cardiopulmonary resuscitation procedure on mice with hypothermic heads (27°C, a condition that protects the CNS from virtually all ischemic damage (Neigh et al., 2004)). Survival, as well as behavioral, histological, inflammatory, and neuroendocrine endpoints were assessed; outcomes were consistently worse in mice that underwent normothermic CA/CPR in the Mid-Light phase relative to those that experienced CA/CPR at different times of the day, thereby indicating that daily variation exists in the susceptibility to ischemic events. We then tested the hypothesis that day-night differences in the circulating concentrations of endogenous melatonin mediated time of day differences in ischemic outcomes; melatonin is a hormones released only during the dark phase that underlies many daily rhythms in physiology and behavior (reviewed in Pandi-Perumal et al., 2006) and both endogenous and exogenous melatonin has neuroprotective properties (Reiter et al., 2005). We also examine potential mediation by daily rhythms in corticosterone. Corticosterone concentrations in rodents increase towards the beginning of the active phase (dark period) peak several hours thereafter and decline through the rest of the day. Circulating corticosterone is an important determinant of ischemic outcomes and can render target tissues more vulnerable to ischemic damage (Bain et al., 1992; Sapolsky and Pulsinelli, 1985). Together, these studies will clarify whether time of day affects ischemic vulnerability.
Methods
Animals
Adult male C3H/e mice were purchased from Harlan (Indianapolis, Indiana). Upon arrival in our laboratory, mice were individually housed and maintained in a 14:10 light dark cycle. All mice had ad libitum access to food (Harlan Teklad #8640) and filtered tap water. All experimental conditions were approved by the Ohio State University Institutional Lab Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
Mice were randomly assigned to undergo CA/CPR at one of three points in the daily cycle, Mid-light (surgeries 6–8 hours after lights on; n=4 histology n=4 rt-PCR), Light→Dark (surgeries within 1 h of lights off; n=5 histology n=6 rt-PCR), and Mid-dark (surgeries 4–6 h after lights off; n=6 histology n=6 rt-PCR) and hypothermic (n=9 histology and n=10 rt-PCR). In order to induce CA/CPR at different subjective times of the day, but still have the surgeries occur during a similar window for the surgeons and animal care staff, mice were housed in ventilated light-tight cabinets with phase-shifted light-dark cycles (see supplementary table 1). After at least 2 weeks in the respective light conditions, mice were briefly anesthetized and a blood sample collected from the retroorbital sinus (approximately 100 μl). Approximately twenty- four hour later, mice underwent either normothermic (head temperature = 37°C) or hypothermic (head temperature = 27°C) cardiac arrest and CPR (see supplementary methods for description of the surgical procedure).
Cardiac Arrest/CPR Procedure
Mice were anesthetized with 3% halothane in air, intubated, and maintained on 1.5% halothane. A temperature probe was placed in the temporalis muscle on the left side of the head. Temporalis muscle temperature was used as an index of brain temperature. A previous study validated this measure in rats (Jiang and North, 1991), and we have previously demonstrated in mice that brain temperature and temporalis temperature are highly correlated (r2 = 0.94) over the range of temperatures experienced during our cardiac arrest/CPR procedure (24 to 39.5°C) (Neigh et al., 2004). Head temperature was manipulated independently of body temperature through the use of a double lumen coil that was placed around the head and filled with circulating water to achieve a brain temperature of 37°C (normothermic), or 27°C (hypothermic). A second temperature probe was placed to monitor rectal temperature. A PE10 catheter was inserted into the right jugular vein for potassium chloride (KCl) and epinephrine (EPI) administration. A cannula (Fine Science, Foster City, CA, USA) was inserted into the right femoral artery, and connected to a blood pressure transducer (Columbus Instruments, Columbus, OH) to allow continuous monitoring of arterial blood pressure. The intubation tube was connected to a ventilator (Columbus Instruments, Columbus, OH, USA) and mice were ventilated with a tidal volume of 150 μl and a respiratory rate of 160 breaths/min. Mice were stabilized for 10 min during which time blood pressure and temperatures were recorded at one min intervals. At the end of the acclimation period, body temperature was decreased to 27° C by circulating cold water through a coil system beneath the animal and placement of an alcohol patch on the ventrum. To induce cardiac arrest, KCl (50 μl, 0.5 M, 4°C) was injected via the jugular catheter. The mice were detached from the ventilator. Slow re-warming via heating lamp and thermal blanket begin when body temperature reached 27°C after approximately 4 min of arrest. At 7 min 45 sec into the arrest period, the mouse was reattached to the ventilator and ventilated with 100% oxygen with a tidal volume of 150 μl and a respiratory rate of 160 breaths/ min. Eight min after injection of KCl, CPR was initiated via injection of epinephrine (16 μg EPI in 0.6 cc saline, 37°C) into the jugular vein catheter and chest compressions (300/min). Additional EPI was administered in increments of 0.5 μg in conjunction with continued chest compressions until the mouse was resuscitated (maximal dose of EPI: 32 μg). Ten minutes after return of spontaneous circulation (ROSC) blood was drawn from the femoral artery catheter (approximately 100 μl). Mice were maintained on 100% oxygen for 25 min following ROSC. Catheters were then removed and skin sutured.
Open Field Testing
Mice were tested in the open field task two days prior to CA/CPR and six days post-reperfusion. The test chamber was enclosed in a sound and light attenuating cabinet that consisted of a 60cm2 clear Plexiglas arena with corncob bedding on the bottom. The arena was surrounded by a series of infrared lights that tracked the movement of the mouse in three-dimensions. Each mouse was tested for 60 min during the dark cycle. The test chamber was cleaned thoroughly with a 70% ethanol solution and the bedding changed between each test. The results were generated online by the PAS software package (San Diego Instruments, San Diego, CA, USA). The total locomotor activity (number of beam breaks), percentage of time spent in the periphery versus the center of the arena were recorded.
Tissue Collection, Processing, and Analysis
During the early portion of the light cycle, seven days post-reperfusion mice were deeply anesthetized, a blood sample was collected from the retroorbital sinus, and then they were given a lethal dose of sodium pentobarbital. Mice were then perfused transcardially with ice-cold 0.1M PBS and 4% paraformaldehyde. Brains were removed, post-fixed for 4 h, cryoprotected in 30% sucrose until they sunk, frozen on crushed dry ice, and then stored at −70° C until they were sectioned at 14μm on a cryostat and thaw-mounted on to Super Frost Plus slides (Fisher, Hampton, NH, USA). Sections were stored at −20° C until further processing.
Fluoro Jade C Histochemistry
Fluoro-Jade C (FJ) is a fluorescein derivative that labels degenerating neurons. Mounted sections were stained according to established protocols (Schmued and Hopkins, 2000a; Schmued and Hopkins, 2000b). Briefly, slides were dried at room temperature, immersed in a basic ethanol solution (80% containing 1% sodium hydroxide) and then rinsed in 70% ethanol and distilled water(dH20). Slides were then treated with potassium permanganate (.06% in (dH20) for 10 minutes, rinsed with water, and then incubated in Fluoro Jade C (0.0001% in a 1% acetic acid solution); sections were simultaneously counterstained with DAPI (Sigma-Aldrich, St. Louis, MO, USA), rinsed in dH20, and thoroughly dried on a slide-warmer, cleared for 1 minute in xylene, and coverslipped with DPX (Sigma).
Fluoro Jade positive cells were counted in multiple hippocampal regions (approximately 2mm caudal to bregma; CA1, CA2, CA3, dentate gyrus, dentate hilus, and subiculum) online by an experimenter unaware of the experimental conditions associated with each sample. Both sides of the hippocampus were counted in a single section and averaged. Black and white images of fluorescently-stained sections were captured with a digital camera (Axiocam, Zeiss) connected to a fluorescent microscope (Zeiss Axioskop) using Axiovision software (Zeiss). Sections with DAPI and Fluoro Jade C were photographed sequentially using appropriate excitation wavelengths (365 and 485 nm respectively), then pseudocolored, superimposed, and merged.
Microglia were visualized using an antibody directed against MAC-1. Slides were air dried, rinsed in distilled water, and then blocked with rabbit serum and bovine serum albumin (BSA). Slides were then incubated for 24 h at room temperature with rat anti-CD11b antibody (Serotec, Raleigh, NC) diluted 1:100 in PB containing 0.3% Triton-X, rabbit serum and BSA. Slides were then rinsed and incubated with rabbit anti-rat secondary antibody (1:500; Vector Labs, Burlingame, CA, USA) for 2 h. Sections were quenched in H202 in methanol and then rinsed and treated with Elite ABC reagent for 60 min. Sections were again rinsed with PBS and then visualized with DAB containing nickel. After visualization, slides were rinsed in distilled H2O, then dehydrated, cleared, and coverslipped.
Photographs of multiple hippocampal regions (CA1, CA2, CA3, dentate gyrus, subiculum, and overlying parietal cortex were taken with a Nikon E800 microscope at 20x. Images were digitized and proportional stained areas were assessed using Image J software (NIH). Briefly, images from each anatomical region were assessed by the software to determine the immunoreactive regions. Then fixed size rectangular `scan boxes' were superimposed over the image and the percentage of stained area within the box was recorded. Proportional area was then expressed as the percentage of the tissue stained relative to the entire size of the scan box. It is important to note that increases in proportional area do not necessarily indicate the presence of greater numbers of microglia, but does provide an assessment of the activational state of this cell type as it becomes hypertrophic in the injured CNS (Coggeshall and Lekan, 1996; Popovich et al., 1997).
Real Time PCR
Twenty-four hours after CA/CPR, fresh brains were collected from an additional 5 animals/time point/head temperature. At that point, mice were euthanized, a trunk blood sample was collected, and then brains were removed using aseptic techniques and stored in RNAlater RNA stabilization solution (Ambion, Austin, TX, USA) overnight at 4°C. Hippocampi were dissected out and total RNA was extracted from >30mg of individual hippocampi using a homogenizer (Ultra-Turrax T8, IKA Works, Wilmington, NC, USA) and an RNeasy Mini Kit according to manufacturer's protocol (Qiagen, Valencia CA, USA). Extracted RNA was suspended in 30 μl RNase-free water and RNA concentration was determined by spectrophotometer (Nanodrop-1000, Nanodrop Technologies, Wilmington, DE, USA). All RNA samples were stored at −70°C until further analysis. cDNA was created via reverse transcription of 2 μg of RNA from each sample with MMLV Reverse Transcriptase enzyme (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol.
Il-1β and Tnfα primers and probes (Overbergh et al., 1999) were synthesized as follows, with probes labeled with 6-FAM (fluorescent dye) and MGB (non-fluorescent quencher) at the 5' and 3' ends, respectively: Il-1β forward 5'-CAACCAACAAGTGATATTCTCCATG-3', Il-1β reverse 5'-AGATCCACACTCTCAGCTGCA-3', Il-1β probe 5'-CTGTGTAATGAAAGACGGCACACCCACC -3'; Tnfα forward 5'-CATCTTCTCAAAATTCGAGTGACAA-3', Tnfα reverse 5'-GGGAGTAGACAAGGTACAACCC-3', Tnfα probe 5'-CACGTCGTAGCAAACCACCAAGTGA -3'. A TaqMan 18S Ribosomal RNA primer and probe set (labeled with VIC fluorescent dye; Applied Biosystems, Foster City, CA, USA) was used as the control gene for relative quantification. Mac-1 and Bcl-2 premade primer probe kits were purchased from Applied Biosystems. Amplification was performed on an ABI 7000 Sequencing Detection System by using Taqman® Universal PCR Master Mix. The universal two-step RT-PCR cycling conditions used were: 50° C for 2 min, 95° C for 10 min, followed by 40 cycles of 95° C for 15 sec and 60° C for 1 min. Relative gene expression of individual samples run in duplicate was calculated by comparison to a relative standard curve and then expressed relative to the ribosomal RNA subunit 18s.
Radioimmunoassay Procedures
The blood samples were collected into heparinized microcapillary tubes and then kept on ice until they were spun for 30 min at 3500rpm. The resultant supernatant was removed and stored at −80°C. All blood samples were assayed for total circulating corticosterone, in a single assay, using a double antibody I125 radioimmunoassay (MP Biomedicals Irvine, CA, USA). The assay was conducted following the guidelines set by the manufacturer. These kits are highly specific and cross reactivity with other steroids is less than 1%. Intra-assay variance was less than 10% and the minimum detection limit was 5 ng/ml.
Luzindole manipulation
In order to determine whether endogenous melatonin mediated the neuroprotective phenotype associated with the Light→Dark and Mid-Dark groups (both normo-and hypothermic) we treated mice with the melatonin receptor antagonist luzindole (30mg/kg in 0.1 ml of 4% ethanol/saline; IP) or its vehicle (0.1 ml EtOH saline) 30 minutes prior to cardiac arrest. This dose was used because it has been previously shown to block immunological responses to exogenous melatonin in house mice (Drazen et al., 2001). Animals were then processed for behavioral and histological responses to ischemia as described above.
Data Analyses
All analyses were first conducted as a 2 × 3 ANOVA for time point (Mid-Light, Light→Dark and Mid-Dark) and head temperature (normothermic vs. hypothermic). Preplanned comparisons were conducted within each head temperature between time points. At no point did hypothermic animals differ across time points and so further analyses were conducted as a four level one-way ANOVAs (group) with the three normothermic time points and all hypothermic animals. Head and body temperature arterial blood pressure, and locomotor activity were all analyzed with repeated measures analyses. If a significant F score was detected, then multiple comparisons were conducted with Tukey's HSD tests. Mean differences were considered statistically significant if p≤0.05.
Results
Normothermic cardiac arrest surgical parameters were similar regardless of the time-of-day at which they occurred (see supplementary figure 1).
Survival
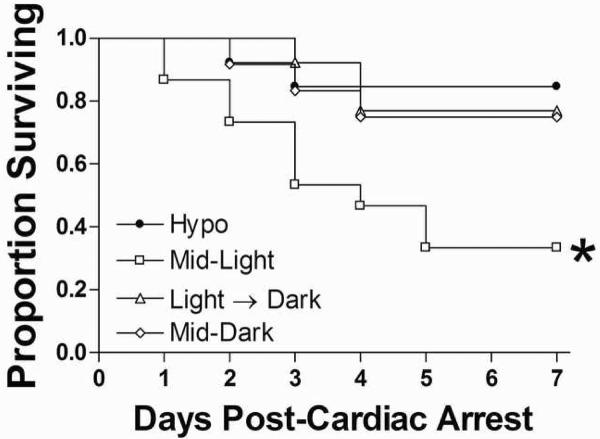
The time of day in which cardiac arrest occurred significantly altered 7-day survival (see figure 1). Eighty-four percent of mice in the hypothermic groups survived CA/CPR. Among the normothermic groups, mice in the Light→Dark and Mid-Dark groups survived at 76.9% and 75%, respectively. In contrast, only 33% of mice that underwent CA/CPR during the light period (Mid-Light group) survived to day 7 post-reperfusion. Thus, timing of CA/CPR significantly altered survival (Kaplan-Meier Survival Analysis; χ2=10.19. Post-hoc analyses indicate that this effect is mediated by the reduction in survival in the Mid-Light group (p<0.05).
Figure 1. Time-of-day determines cardiac arrest survival.
Cardiac arrest reduced survival in normothermic mice that underwent CA/CPR during the Mid-Light period. Eighty-four percent of mice in the hypothermic groups survived CA/CPR. Among the normothermic groups, mice in the Light→Dark transition group and the Mid-Dark group survived at 76.9% and 75%, respectively. In contrast, only 33% of mice that underwent CA/CPR during the light period (Mid-Light group) survived to day 7 post-reperfusion. Thus, timing of CA/CPR significantly altered survival (Kaplan-Meier Survival Analysis; χ2=10.19, p<0.05). *Significantly different from all other groups at p<0.05.
Behavioral Outcomes
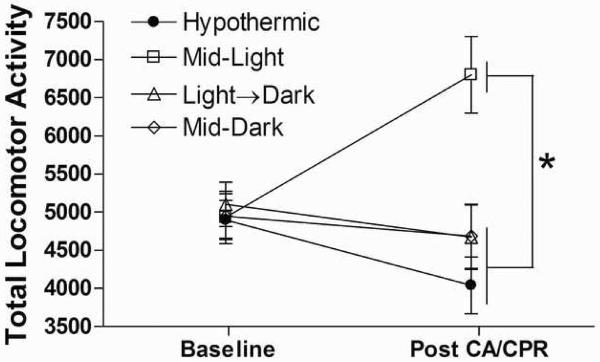
Cardiac arrest-induced hyperactivity was observed only in mice that underwent cerebral ischemia during the Mid-Light period. Post-ischemic hyperactivity has been positively correlated with CA1 neuronal loss and impairs spatial memory by blocking formation of cognitive maps (Wang and Corbett, 1990) that is increased exploration may represent an inability to remember what had been previously investigated. Repeated measures analysis did not detect an overall effect of cardiac arrest on total locomotor activity (F1, 24=.119, p>0.05; see figure 2). However, there was a significant interaction between cardiac arrest and group (F3, 24=6.17, p<0.01) that was mediated by a significant increase in total locomotor activity in the Mid-Light group following cardiac arrest (p<0.05). Conversely, central tendency in the open field was reduced by cardiac arrest (F1, 23=144.42, p<0.0001; data not shown), but was not altered by surgical time of day (p>0.05). Avoidance of the center of an open field chamber can be considered indicative of an anxiety-like state as more anxious rodents tend to prefer the areas close to the outer walls of an unfamiliar space (Crawley, 2000). Therefore it appears that normothermic CA/CPR induces an anxiety-like state independent of surgical timing.
Figure 2. Time-of-day determines the behavioral consequences of cardiac arrest.
Cardiac arrest-induced behavioral hyperactivity was observed only in mice that underwent cerebral ischemia during the Mid-Light period. * Mid-Light differs from all other groups post CA/CPR (p<0.05; data are presented as mean ±SEM). n=6–10/group.
Histology
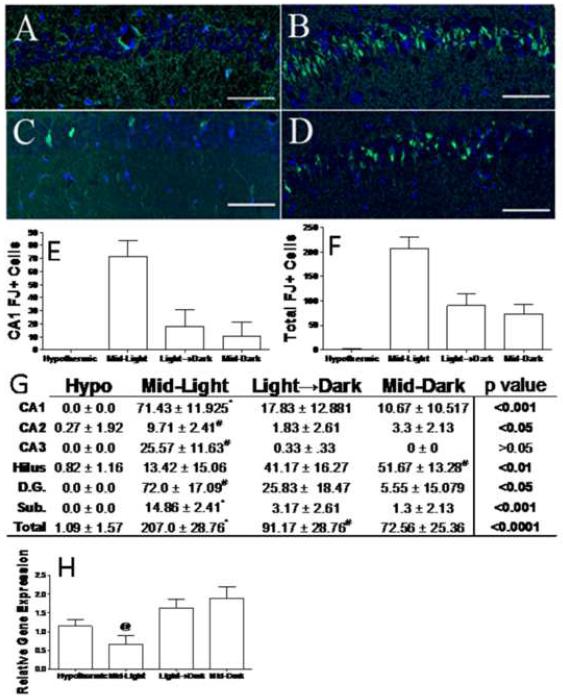
There were essentially no damaged neurons in mice that underwent hypothermic CA/CPR, regardless of time-of-day. In contrast, mice that underwent normothermic cardiac arrest during the Mid-Light period exhibited greater hippocampal damage than all other groups. Cell death data are presented in figure 3. Normothermic cardiac arrest induced hippocampal neuron damage as indicated by FluoroJade staining (figure 3a–d). In both the CA1 region (F3, 39=3.56, p<0.05; see figure 4e) and across the whole hippocampus there was a main effect of group on FJ+ cells (F3, 39=98.99, p<0.0001; see figure 3f). In both the entire hippocampus and CA1 regions, these effects were mediated by greater damage to the Mid-Light animals than any other group (p<0.05 in all cases). Interestingly, Mid-dark mice were the only group with statistically significant numbers of dead FJ+ neurons in the dentate hilus (p<0.05). However, in four other regions, (the CA2 and CA3 subfields, dentate gyrus and subiculum) only Mid-light mice had greater numbers FJ+ neurons than did hypothermic controls. The early post-ischemic mRNA expression of the anti-apoptotic protein BCL-2 was also decreased in mice that underwent CA/CPR during the middle of the light period (F3, 22=4.32, p<0.05 see figure 3h).
Figure 3. Time-of-day determines that cell death responses to ischemia.
Representative sections of Fluoro-jade C (degenerating neurons; green) and DAPI (nuclear counterstain; blue) stained tissue from the CA1 region of mice that underwent hypothermic CA/CPR (a) or normothermic CA/CPR during the Mid-Light (b), Light-Dark transition (c), or Mid-Dark period (d). Quantification of Fluoro Jade positive neurons in the CA1 field (e) and across the whole hippocampus (f). Table of mean (±SEM) for Fluoro-jade positive neurons in selected regions (g). Hippocampal bcl-2 gene expression relative to 18s rRNA gene expression at 24 h post-CA/CPR (h). Scale bar =100μm. CA -Cornu Ammonis, D.G. dentate gyrus, Sub. subiculum. * indicates significantly different from all other groups at p<0.05; # indicates significantly different from hypothermic mice at p<0.05. n=7–11/group for histology and 4–10/group for PCR.
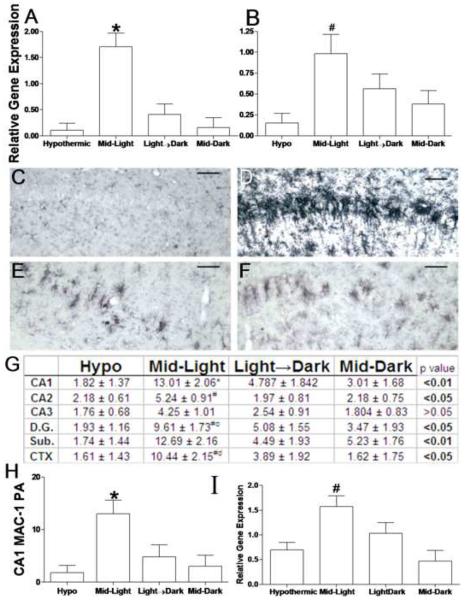
Figure 4. Time-of-day determines inflammatory responses to cardiac arrest.
Proinflammatory cytokine gene expression was potentiated by CA/CPR during the Mid-Light. (a) Il-1β and (b) Tnfα mRNA gene expression relative to 18s rRNA. Microglial activation was also potentiated in mice that underwent ischemia during the middle of the light period. Representative sections of MAC-1 stained sections through the CA1 field of the hippocampus in mice that underwent hypothermic CA/CPR (c) or normothermic CA/CPR in the Mid-Light (d), Light→ Dark Transition (e) or in the Mid-Dark (f). Table of proportional areas of microglial activation across the hippocampus and associated regions (g). (h) MAC-1 proportional area in the CA1 field. (i) Immunohistochemical evidence of increased microglial activation was confirmed with MAC-1 rat-PCR 24 h post-CA/CPR. Data are presented as the mean ±SEM. * significantly different from all other groups (p<0.05); # significantly different from hypothermic mice (p<0.05); c significantly different from mid-dark mice (P<0.05); d significantly different from Light→Dark mice (p<0.05). Scale bar =100μm. CA-Cornu Ammonis, D.G. dentate gyrus, Sub. subiculum, CTX, posterior parietal cortex n=7–11/group for histology and 4– 10/group for PCR.
Inflammatory Responses
A separate cohort of animals underwent cardiac arrest as described above, but were euthanized 24 h following cardiac arrest for the assessment of early gene expression changes. Real-time PCR analysis of the mRNA encoding the proinflammatory cytokines interleukin-1β (IL-1β indicated that the four groups exhibited differential Il-1β mRNA expression (F3, 26= 10.30, p<0.0001; see figure 4a). This effect was mediated by elevated expression in the hippocampi of mice that underwent normothermic CA/CPR during the Mid-light period (p<0.05 in all cases). No other normothermic group differed from hypothermic in Il-1β gene expression. Tnfα expression also differed across groups (F3, 22=4.01, p<0.05; see figure 4b). The normothermic Mid-light mice were the only group that had greater Tnfα expression than hypothermic mice, although there were no significant differences among the normothermic animals. Importantly, no other normothermic group exhibited significantly elevated (relative to hypothermic animals) cytokine gene expression (p>0.05 in all cases).
Microglial data are summarized in figure 4c–g. There was an overall main effect of group on microglial activation in the ischemia vulnerable CA1 region, as assessed by the proportional area of MAC-1 staining (F3, 20=7.23, p<0.01). This effect primarily reflects a large increase in microglial activation in Mid-light mice relative to all other groups (p<0.05 in all cases; summarized in 4g). No other normothermic group was significantly different from hypothermic mice (p>0.05 in all cases). In the CA2, dentate gyrus, and overlying parietal cortex there was significant microglial activation (relative to hypothermic mice) only in the mid-light group. Importantly, there was no statistically significant microglial activation in any brain region of mice that underwent normothermic CA/CPR during the Light-Dark transition or in the Mid-Dark when compared to hypothermic animals. We also confirmed histological evidence of microglial activation with rt-PCR; animals that underwent cardiac arrest during the Mid-Light period had significantly greater Mac-1 gene expression (immunohistochemical antigen used to detect microglia) 24 h following cardiac arrest relative to all other groups (F3,22=6.21, p<0.01; see figure 4h).
Glucocorticoids
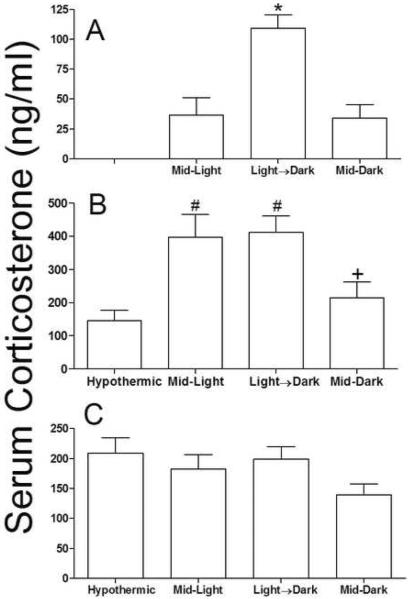
Basal glucocorticoids concentrations were altered by the phase of sampling (F2,27 = 13.20, p<0.0001; see figure 5a), an effect that was mediated by increased circulating corticosterone when mice were sampled during the Light→Dark transition (p<0.05, figure 5a). A separate subset of mice underwent cardiac arrest and were euthanized 24 h post-reperfusion, but in the same time-of-day as the surgery had occurred; groups differed significantly in circulating corticosterone at this time point (F3, 22=11.32, p<0.0001; see figure 5b) as both Mid-Light and Light→Dark groups had elevated glucocorticoids relative to hypothermic mice (p>0.05 in both cases). Circulating corticosterone in the early portion of the inactive (lights on) period seven days after cardiac arrest did not differ among groups (p>0.05; see figure 5c)
Figure 5. Circulating glucocorticoids may not explain time-of-day differences in ischemic outcome.
Serum corticosterone (a) 24 h prior to CA/CPR, (b) 24 h post-reperfusion or (c) seven days post-reperfusion. Data are presented as the mean ±SEM. *Significantly different from all other groups (P<0.05). #Significantly different from hypothermic mice (p<0.05). + Significantly different than Light→Dark (p<0.05). n= 4–10/group.
Melatonin manipulation
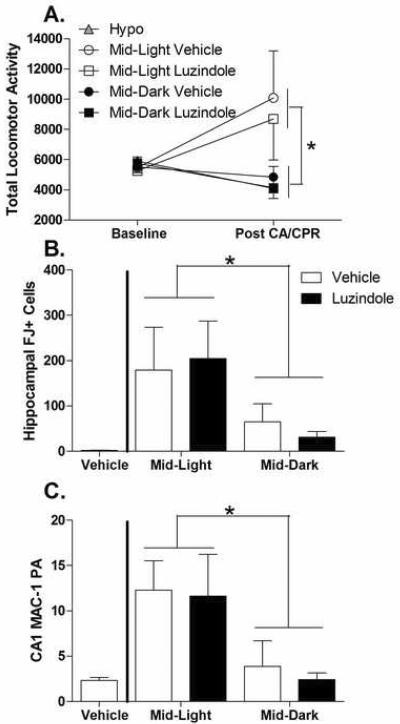
This experiment replicated the main time-of-day findings of the initial experiment and revealed no evident role for melatonin receptors in mediating the time-of-day differences in ischemic outcome. There were no statistically significant differences observed among the hypothermic groups either by time-of-day or drug treatments and so they were collapsed for further analyses. Normothermic cardiac arrest did not significantly alter locomotor behavior (F1, 13=1.575, p>0.05; see figure 6a) across all animals however there was an increase in total locomotor activity in mice that underwent CA/CPR during the middle of the light (F1, 13=5.623, p<0.05). There was no effect of drug treatment (F1, 13=0.19, p>0.05) or an interaction with between the drug and time of day. Regardless of drug treatment, cardiac arrest resulted in significantly more FJ+ neurons in the CA1 (F1,17=5.25, p<0.05) and across the whole hippocampus (F1,17=5.96, p<0.05; see figure 6b) when induced among the normothermic animals in the middle of the light period compared to the middle of the dark period. Likewise, microglial responses to cardiac arrest in the CA1 were enhanced by surgery during the light compared to the dark period (F1, 17=9.69, p<0.01; figure 6c) and there was no effect of drug treatment (F1, 17=0.14, p>0.05) or an interaction between time of day and drug treatment.
Figure 6. Melatonin receptor antagonists do not alter time-of-day differences in ischemic outcome.
Treatment with luzindole 30 minutes prior to CA/CPR did not alter time of day differences in (A) post-ischemic hyperactivity (B), or time-of-day differences in FJ+ cells throughout the hippocampus (C) and microglial activation in the CA1 field. p> 0.05 in each comparison between luzindole and vehicle. Data are presented as the mean ± SEM. FJ- Fluoro Jade. *indicates significantly differences between the Mid-Light and Mid-dark groups. n=7–10/group.
Discussion
Ischemic outcomes vary according to the time of day when the injury occurred. In our study, mice that underwent CA/CPR during the middle of the light period survived at a significantly lower rate, and those that survived displayed increased Bcl-2 mRNA expression, elevated microglial activation, increased proinflammatory cytokine gene expression, more degenerating hippocampal neurons, and behavioral abnormalities, relative to mice that experienced cardiac arrest at other times of the day. One particularly salient result of this study is that a difference of only a few hours in the onset of cerebral ischemia dramatically affects the pathophysiological processes that evolve for several days after successful resuscitation. Although the first signs of neuronal death are delayed many hours or days after cardiac arrest, the temporal window in which the outcome trajectories are established is relatively narrow (Kirino et al., 1984; Pulsinelli, 1985). This is illustrated by the reduced expression of the anti-apoptotic bcl-2 gene at 24-hours post reperfusion, which occurred several days before histological evidence of neuron loss. BCL-2 is expressed at very low levels in healthy adult brains, but is up-regulated following neuronal injury, ultimately serving as an endogenous neuroprotectant (Bergeron and Yuan, 1998). Whereas bcl-2 over-expression protects against neuronal loss after ischemia (Kitagawa et al., 1998; Martinou et al., 1994; Zhao et al., 2003) a decrease in bcl-2 expression is associated with increased neuronal death (Chen et al., 2000; DeVries et al., 2001). Thus, reduced post-ischemic hippocampal Bcl-2 gene expression among mice in the Mid-Light group may have predisposed them to increased neuronal death.
In common with humans, mice exhibit daily fluctuation in susceptibility to CA. Previous studies have provided indirect evidence for daily fluctuations of ischemic sensitivity as indicated by differential induction of caspase proteins following cardiac arrest across the day (Tischkau et al., 2007) although they reported the greatest indication of neuronal damage during the dark phase. Conversely, survival and the incidence of hemorrhage after traumatic brain injury also varied across the day but with more damage during the light period (Martinez-Vargas et al., 2006). In our study, the timing of the increased tissue susceptibility to ischemia in mice (mid-light; the middle of the inactive period for nocturnal mice) is different than the period during which most CA occur in humans (beginning of the active period for putatively diurnal humans). However, the methods for determining maximal susceptibility in these two types of studies differ; in humans it is difficult to differentiate between factors that affect the incidence of CA, such as fluctuations in BP, HR, and sympathetic tone, and those that may affect the severity of CA, such as neuroinflammation. In contrast, this mouse study uses a standardized procedure to induce CA, and then focuses on the physiological processes that result. Nonetheless, these murine data provide evidence that brain tissue displays fluctuations in susceptibility to ischemia and that a difference of just a few hours in onset time can significantly affect mortality and functional recovery among those mice that survive.
It is tempting to speculate that the enhanced inflammatory responses (microglial activation and cytokine gene expression) drive the time of day variation in ischemic outcomes. The deleterious effects of inflammatory responses in the CNS following ischemia are well documented (Stoll et al., 1998; Streit et al., 1988; Yrjanheikki et al., 1998) (but see (Hayashi et al., 2006)) and Mid-Light mice exhibited increased proinflammatory cytokine gene expression (Il-1β and Tnfa), greater microglial activation and increased cellular damage. The increase in inflammatory responses in Mid-Light mice (or potentially the dampening of inflammation at other times of the day) could be the proximate mediator of poorer outcomes in Mid-Light mice. In addition, expression of each gene we examined was equivalent across the hypothermic groups, thereby suggesting that in the absence of neuronal damage time-of-day did not influence Il-1β, Tnfα, BCL-2, or Mac-1 expression in the hippocampus (supplementary table 2).
The second study addressed whether melatonin was the mechanism through which time of day influenced neuroinflammation and neuronal damage after CA/CPR. Melatonin is a neuroendocrine transducer of time information and a potential modulator of ischemic outcomes (Poeggeler, 2005). Because melatonin is secreted from the pineal gland during the dark, the only group that would not have pineal melatonin in circulation (and in the CSF) at the time of CA\CPR is the mid-light group. Although melatonin has long been studied for its role in biological timing, it is now becoming apparent that melatonin is a potent antioxidant (Poeggeler et al., 1993) and can act as a protective factor against ischemic damage in a number of different organs and tissues (Cuzzocrea and Reiter, 2001; Reiter et al., 2005). The protective effects of melatonin have been attributed to its potent antioxidant properties that acts both via receptor-dependent mechanisms to upregulate gene expression of antioxidant enzymes and directly via scavenging of free radicals (Antolin et al., 1996; Barlow-Walden et al., 1995; Reiter et al., 2005). In addition, repeated treatment with high doses of melatonin enhances ischemic induction of BCL-2 after experimental stroke (Sun et al., 2002). In the CNS, short-term treatment with physiological or pharmacological doses of exogenous melatonin reduces cell death, oxidative stress, and functional deficits in both focal and global ischemia models (Kilic et al., 1999; Letechipia-Vallejo et al., 2001; Manev et al., 1996; Sun et al., 2002) (Stehle et al., 2002). However, administration of the melatonin receptor antagonist luzindole just prior to ischemia did not affect behavioral or histological outcomes. It seems probable that melatonin receptor activation (particularly MT1 as luzindole has a 10 fold greater affinity for MT1 than MT2) prior to and during the surgery are not necessary for time-of-day differences in ischemic outcomes. Neuroprotective effects of melatonin that are independent of receptor activation have been reported and attributed to direct free radical scavenging by melatonin and a similar phenomenon cannot be ruled out here (Cazevieille et al., 1997). If endogenous pineal melatonin was responsible for protection against ischemic damage during the night, than we would expect that luzindole would exacerbate ischemic outcomes during the dark but not light phases. Future studies are planned to investigate the physiological transduction of time-of-day information as it appears to be independent of melatonin receptor signaling. One such signaling molecule is corticosterone, an important mediator of neuronal death following ischemia (DeVries et al., 2001; Sapolsky and Pulsinelli, 1985) that displays a well-characterized daily rhythm. We had hypothesized that daily fluctuations in circulating glucocorticoids could underlie time-of-day differences in ischemic outcomes in our study. Circulating cortisol concentrations are predictive of infarct size and mortality following myocardial infarction (Bain et al., 1992; Stubbs et al., 1999), in humans, and glucocorticoids can exacerbate the neuronal injury following global and focal ischemia (DeVries et al., 2007; DeVries et al., 2003) at least in part via suppression of bcl-2 expression (DeVries et al., 2001). However, it is unlikely that pre-ischemic, intra-ischemic, or post-ischemia glucocorticoid concentrations mediate the time-of-day effects on ischemic outcomes in this study because there was no obvious relationship between corticosterone concentrations and extent of neuronal death. For example, circulating corticosterone concentration was higher during the surgical window for mice in the Light→Dark group than the other two groups (Mid-Light and Mid-Dark), but the Light→Dark and Mid-Dark groups displayed similar levels of neuronal damage and neuroinflammation. Likewise, pre-ischemic corticosterone concentrations were similar for the Mid-Light and Mid-Dark groups, but they exhibited very different outcomes. Also, corticosterone content was similar among all groups at reperfusion and at 24 h post-ischemia. Thus, circulating corticosterone does not appear to mediate the time-of-day differences in ischemic outcome.
Therapeutic hypothermia is a powerful neuroprotectant that can protect neurons from ischemic cell death via inhibition of inflammatory responses, induction of neuroprotectant genes and growth factors, and a slowing of overall cellular metabolism and excitotoxic transmission(Liu and Yenari, 2007). The hypothermia model used in this study was apparently so profoundly neuroprotective that it was not possible to determine if there are daily rhythms in the extent to which hypothermia is neuroprotective. However, it seems likely that the efficacy of various neuroprotectant strategies will vary across the day and the interaction between hypothermia and daily rhythms in ischemic vulnerability are likely to be instructive.
The differential survival of animals that underwent cardiac arrest at different times of the day is likely mediated by greater neuronal damage as this effect can be blocked by lowering head temperature. As the animals with the most ischemic damage are presumably the ones most likely to die the differences in behavioral and physiological outcomes between animals that underwent cardiac arrest at different times of the day underestimated.
Together, these data indicate that CNS tissue undergoes endogenous fluctuations in susceptibility to ischemia-induced inflammation and cell death. Although a single specific mechanism driving time-of-day differences in ischemic outcomes was not indentified in this experiment it seems probable that a number of signaling pathways both communicate daylength information to target tissues and participate in the fluctuations in vulnerability, future studies will continue to search for those mediators. In any case, a role for the MT-1 melatonin receptor has been ruled out, but it remains possible that this rhythm in neuronal susceptibility is coordinated by the endogenous melatonin rhythm. Daily rhythms in almost all physiological processes are coordinated by the master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus, which in turn is entrained primarily by photic cues. The role of the SCN in determining ischemic outcome has not previously been examined. In any case, improved understanding of the endogenous rhythms that influence survival and recovery from cerebral ischemia could help explain some of the variability in ischemic outcome that exists after controlling for traditional variables, and may in turn identify novel therapeutic targets and provide basic insights into the physiological consequences of daily rhythmicity.
Supplementary Material
Acknowledgments
The authors thank Kathleen Broderick, Jonathon Wade, Stephanie Kidder and M. Sima Finy for excellent technical assistance and Drs, Holly Chalk, Lynn Martin and Camille Fontaine, for helpful discussions of the data and comments on earlier versions of the manuscript. We also thank Dr. Georgia Bishop for technical assistance and the use of her microscope. This research was supported by NSF grant IBN 04-16897 (RJN), NIH grants MH 57535 (RJN), NS40267, and HL080249 (ACD), The American Heart Association (EIA award to ACD) and The Ohio State University Presidential Fellowship (ZMW). Additional support was provided by NIH P30NS045758.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe M, et al. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–6. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antolin I, et al. Neurohormone melatonin prevents cell damage: effect on gene expression for antioxidant enzymes. Faseb J. 1996;10:882–90. doi: 10.1096/fasebj.10.8.8666165. [DOI] [PubMed] [Google Scholar]
- Bain RJ, et al. Serum cortisol levels predict infarct size and patient mortality. International Journal of Cardiology. 1992;37:145–50. doi: 10.1016/0167-5273(92)90201-d. [DOI] [PubMed] [Google Scholar]
- Barlow-Walden LR, et al. Melatonin stimulates brain glutathione peroxidase activity. Neurochemistry International. 1995;26:497–502. doi: 10.1016/0197-0186(94)00154-m. [DOI] [PubMed] [Google Scholar]
- Becker LB, et al. Outcome of CPR in a large metropolitan area--where are the survivors? Annals of Emergency Medicine. 1991;20:355–61. doi: 10.1016/s0196-0644(05)81654-3. [DOI] [PubMed] [Google Scholar]
- Bergeron L, Yuan J. Sealing one's fate: control of cell death in neurons. Current Opinion in Neurobiology. 1998;8:55–63. doi: 10.1016/s0959-4388(98)80008-1. [DOI] [PubMed] [Google Scholar]
- Cazevieille C, et al. Melatonin protects primary cultures of rat cortical neurones from NMDA excitotoxicity and hypoxia/reoxygenation. Brain Research. 1997;768:120–124. doi: 10.1016/s0006-8993(97)00611-2. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Suppression of Endogenous bcl-2 Expression by Antisense Treatment Exacerbates Ischemic Neuronal Death. Journal of Cerebral Blood Flow & Metabolism. 2000;20:1033–1039. doi: 10.1097/00004647-200007000-00002. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cohen MC, et al. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. American Journal of Cardiology. 1997;79:1512–6. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What's Wrong with My Mouse?: Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley-Liss; New York: 2000. [Google Scholar]
- Cuzzocrea S, Reiter RJ. Pharmacological action of melatonin in shock, inflammation and ischemia/reperfusion injury. Eur J Pharmacol. 2001;426:1–10. doi: 10.1016/s0014-2999(01)01175-x. [DOI] [PubMed] [Google Scholar]
- DeVries AC, et al. 2006 Curt P. Richter award winner: Social influences on stress responses and health. Psychoneuroendocrinology. 2007;32:587–603. doi: 10.1016/j.psyneuen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- DeVries AC, et al. Social modulation of stress responses. Physiol Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- DeVries AC, et al. Social stress exacerbates stroke outcome by suppressing Bcl-2 expression. Proceedings of the National Academy of Sciences of the United States of America; 2001. pp. 11824–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen DL, et al. Melatonin enhancement of splenocyte proliferation is attenuated by luzindole, a melatonin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1476–1482. doi: 10.1152/ajpregu.2001.280.5.R1476. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, et al. The intra-arterial injection of microglia protects hippocampal CA1 neurons against global ischemia-induced functional deficits in rats. Neuroscience. 2006;142:87–96. doi: 10.1016/j.neuroscience.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Herzog ED. Neurons and networks in daily rhythms. Nature Reviews: Neuroscience. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- Ishiura M, et al. Expression of a Gene Cluster kaiABC as a Circadian Feedback Process in Cyanobacteria. Science. 1998;281:1519–1523. doi: 10.1126/science.281.5382.1519. [DOI] [PubMed] [Google Scholar]
- Jastremski M, et al. Glucocorticoid treatment does not improve neurological recovery following cardiac arrest. Brain Resuscitation Clinical Trial I Study Group. JAMA : the journal of the American Medical Association. 1989;262:3427–30. [PubMed] [Google Scholar]
- Jiang ZG, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. Journal of Physiology (London) 1991;443:533–53. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic E, et al. Pinealectomy aggravates and melatonin administration attenuates brain damage in focal ischemia. J Cereb Blood Flow Metab. 1999;19:511–6. doi: 10.1097/00004647-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Kirino T, et al. Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathologica. 1984;64:139–47. doi: 10.1007/BF00695577. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, et al. Amelioration of Hippocampal Neuronal Damage After Global Ischemia by Neuronal Overexpression of BCL-2 in Transgenic Mice. Stroke. 1998;29:2616–2621. doi: 10.1161/01.str.29.12.2616. [DOI] [PubMed] [Google Scholar]
- Kofler J, et al. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Krause GS, et al. Ischemia, resuscitation, and reperfusion: mechanisms of tissue injury and prospects for protection. American Heart Journal. 1986;111:768–80. doi: 10.1016/0002-8703(86)90114-6. [DOI] [PubMed] [Google Scholar]
- Letechipia-Vallejo G, et al. Neuroprotective effect of melatonin on brain damage induced by acute global cerebral ischemia in cats. Archives of Medical Research. 2001;32:186–92. doi: 10.1016/s0188-4409(01)00268-5. [DOI] [PubMed] [Google Scholar]
- Liu L, Yenari M. Therapeutic hypothermia: Neuroprotective mechanisms. Front Biosci. 2007;12:816–825. doi: 10.2741/2104. [DOI] [PubMed] [Google Scholar]
- Manev H, et al. Increased brain damage after stroke or excitotoxic seizures in melatonin-deficient rats. Faseb J. 1996;10:1546–51. doi: 10.1096/fasebj.10.13.8940301. [DOI] [PubMed] [Google Scholar]
- Martinez-Vargas M, et al. Recovery after a traumatic brain injury depends on diurnal variations: Effect of cystatin C. Neuroscience Letters. 2006;400:21–24. doi: 10.1016/j.neulet.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Martinou JC, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13:1017–30. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Muller JE, et al. Circadian variation in the frequency of sudden cardiac death. Circulation. 1987;75:131–8. doi: 10.1161/01.cir.75.1.131. [DOI] [PubMed] [Google Scholar]
- Neigh GN, et al. Cardiac arrest/cardiopulmonary resuscitation increases anxiety-like behavior and decreases social interaction. Journal of Cerebral Blood Flow & Metabolism. 2004;24:372–82. doi: 10.1097/01.WCB.0000112323.75217.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbergh L, et al. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA. Mechanisms of neuroprotection during ischemic preconditioning: lessons from anoxic tolerance. Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology. 2007;147:291–9. doi: 10.1016/j.cbpa.2006.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeggeler B. Melatonin, aging, and age-related diseases: perspectives for prevention, intervention, and therapy. Endocrine. 2005;27:201–12. doi: 10.1385/ENDO:27:2:201. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, et al. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: a hypothesis. J Pineal Res. 1993;14:151–68. doi: 10.1111/j.1600-079x.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Popovich PG, et al. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–64. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Lemmer B. Chronobiology and chronotherapy of ischemic heart disease. Advanced Drug Delivery Reviews. 2007;59:952–965. doi: 10.1016/j.addr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. Prog Brain Res. 1985;63:29–37. doi: 10.1016/S0079-6123(08)61973-1. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, et al. When melatonin gets on your nerves: its beneficial actions in experimental models of stroke. Experimental Biology and Medicine (Maywood) 2005;230:104–17. doi: 10.1177/153537020523000205. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annual Review of Physiology. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Rocco MB, et al. Circadian variation of transient myocardial ischemia in patients with coronary artery disease. Circulation. 1987;75:395–400. doi: 10.1161/01.cir.75.2.395. [DOI] [PubMed] [Google Scholar]
- Roine RO, et al. Neuropsychological sequelae of cardiac arrest. JAMA : the journal of the American Medical Association. 1993;269:237–42. [PubMed] [Google Scholar]
- Sapolsky RM, Pulsinelli WA. Glucocorticoids potentiate ischemic injury to neurons: therapeutic implications. Science. 1985;229:1397–400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000a;874:123–30. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade: novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol Pathol. 2000b;28:91–9. doi: 10.1177/019262330002800111. [DOI] [PubMed] [Google Scholar]
- Stehle JH, et al. Organisation of the circadian system in melatonin-proficient C3H and melatonin-deficient C57BL mice: a comparative investigation. Cell and Tissue Research. 2002;309:173–182. doi: 10.1007/s00441-002-0583-2. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, et al. Entrainment of the Circadian Clock in the Liver by Feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Stoll G, et al. Inflammation and glial responses in ischemic brain lesions. Prog Neurobiol. 1998;56:149–71. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Streit WJ, et al. Functional plasticity of microglia: a review. Glia. 1988;1:301–7. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Stubbs PJ, et al. Circulating stress hormone and insulin concentrations in acute coronary syndromes: identification of insulin resistance on admission. Clinical Science (London) 1999;96:589–95. doi: 10.1042/cs0960589. [DOI] [PubMed] [Google Scholar]
- Sun FY, et al. Neuroprotection by melatonin against ischemic neuronal injury associated with modulation of DNA damage and repair in the rat following a transient cerebral ischemia. Journal of Pineal Research. 2002;33:48–56. doi: 10.1034/j.1600-079x.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- Thompson DR, et al. Time of onset of chest pain in acute myocardial infarction. International Journal of Cardiology. 1985;7:139–48. doi: 10.1016/0167-5273(85)90354-7. [DOI] [PubMed] [Google Scholar]
- Tischkau SA, et al. Time-of-day affects expression of hippocampal markers for ischemic damage induced by global ischemia. Experimental Neurology. 2007;208:314–322. doi: 10.1016/j.expneurol.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Wang D, Corbett D. Cerebral ischemia, locomotor activity and spatial mapping. Brain Res. 1990;533:78–82. doi: 10.1016/0006-8993(90)91798-l. [DOI] [PubMed] [Google Scholar]
- Willich SN, et al. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. American Journal of Cardiology. 1987;60:801–6. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, et al. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proceedings of the National Academy of Sciences of the United States of America; 1998. pp. 15769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, et al. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. Journal of Neurochemistry. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.