Fig. 1.
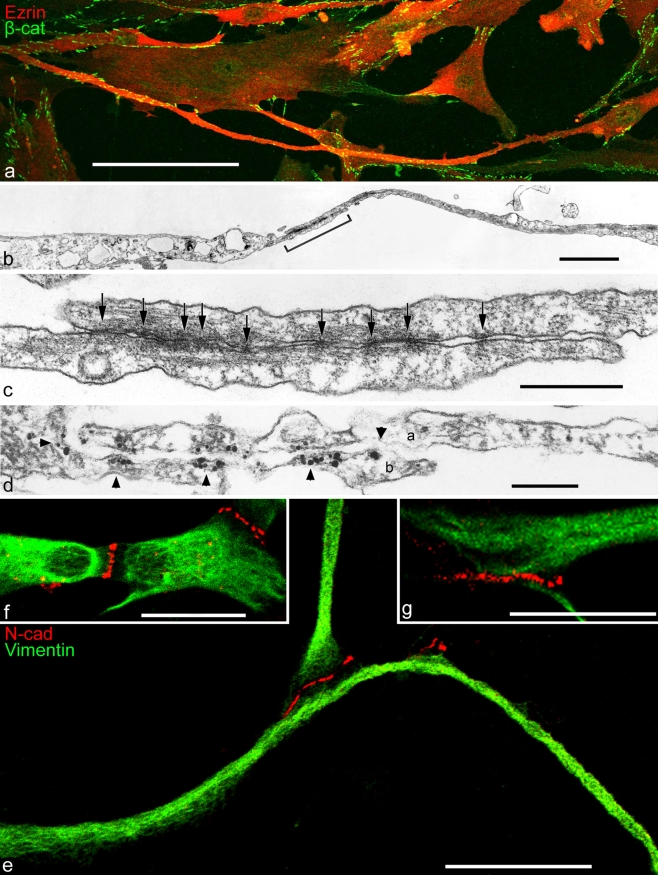
Double-label immunofluorescence (a, e-g) and electron (b, c) and immunoelectron (d) microscopy showing cell processes of cultured human mesenchymal stem cells (MSCs), originally isolated from bone marrow (a-d) or ovine cardiac valve matrix (e-g). a Note that some of the cell processes are extremely long. The giant process extending in the lower part, for example, exceeds 450 µm in length and forms adherens junctions (AJs) of the puncta adhaerentia minima type (PAM) with at least five other cells. The microfilament-rich cell process is immunostained for the actin-binding protein, ezrin (Ezrin, red), and the numerous AJs have reacted with antibodies specific for β-catenin (β-cat, green). b Electron micrograph of the overlapping contact region of two cytoplasmic MSC processes that partly overlap in the contact region (bracket). c Higher magnification of the contact region demarcated in b showing a series of extremely small PAM (arrows; e.g., the diameter of the junction denoted by the arrow right is below 40 nm). d Immunoelectron microscopy of a similar region as that shown in c showing an overlap contact of processes of two cells (a, b); the processes are studded with PAM decorated with silver-enhanced immunogold-label for β-catenin (arrowheads). For details, see Wuchter et al. (2007). e–g Clusters of AJs at the tips of cell processes of cardiac valvular interstitial cells as visualized by immunostaining with antibodies to N-cadherin (N-cad; for details, see Barth et al. 2009). N-cadherin-positive (red) AJs connecting valvular interstitial cells (green, vimentin) are present as terminal punctate clusters at the tips of filopodium-like processes (e.g., the segment shown bottom in e exceeds 100 µm in length). Note the clusters of small AJs connecting the central bodies of three valvular interstitial cells (f) and the relatively large region densely studded with AJs connecting the terminal portions of two cell processes (g). For details, see Barth et al. 2009. Bars 100 µm (a), 2 µm (b), 0.5 µm (c), 0.2 µm (d), 25 µm (e, f), 20 µm (g)