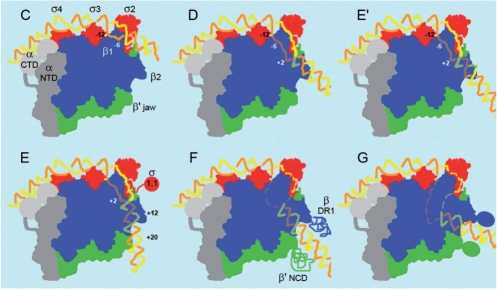
Figure 7.
Proposed structures for the key intermediates in the pathway of formation of a transcriptionally active complex on the wild type T7A1 promoter at 37°C. The model of the open complex proposed by Darst and co-workers from the crystal structure of the Thermus aquaticus RNAP was used as a starting point to create these images (17). α-CTDs and NTDs are shown in light and dark grey respectively. β and β′ are in blue and green, respectively, while sigma is in red. The template strand is in orange and the nontemplate strand in yellow. The shaded parts of the DNA are those that have entered the active site channel and the jaws and are therefore placed behind the β subunit. These intermediates correspond to those shown in Figure 5a, C through G. Following the formation of early complexes stabilized by the interaction of the α-CTDs with the UP-element and σ region 4 with the –35 region of the promoter (A, B and B′ not shown) the DNA is bent towards σ regions 3 and 2 where contacts are made with the spacer and the upstream end of the –10 region, C. The E′ intermediate corresponds to the off-pathway complex. While the pattern of protection in complexes E, F and G does not change, the extent of protection increases as the complex isomerizes into a transcriptionally active structure where the template strand is placed at the active site (G). Protection of the DNA down to p20 is likely due to an interaction with the β DR1 and β′ NCD and their subsequent folding stabilizing the transcriptionally active complex (3,54).