Table 1.
Pd-catalyzed hydroarylation of o-alkynyl biaryls.a
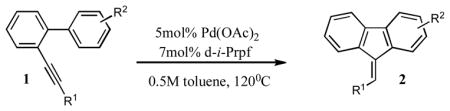 | ||||||||
|---|---|---|---|---|---|---|---|---|
| # | Product | Time, h Yield, %b | # | Product | Time, h Yield%b | # | Product | Time, h Yield, %b |
| 1 |
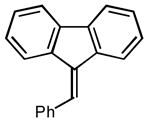 2a |
2.5 98 |
7 |
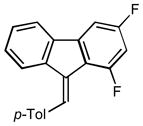 2g |
4.0 95 |
13 |
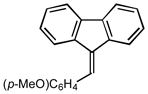 2m |
5.0 87 |
| 2 |
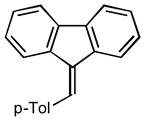 2b |
3.0 95 |
8 |
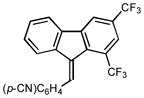 2h |
1.0 93 |
14 |
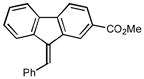 2n |
1.0 85 |
| 3 |
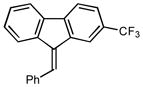 2c |
1.5 96 |
9 |
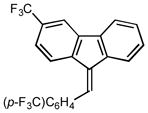 2i |
0.5 98 |
15 |
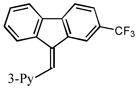 2o |
1.5 77 |
| 4 |
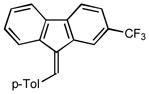 2d |
2.0 96 |
10 |
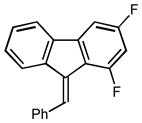 2j |
3.0 93 |
16 |
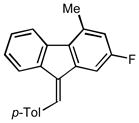 2p |
24 47 |
| 5 |
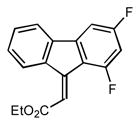 2e |
0.5 79 |
11 |
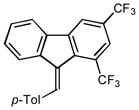 2k |
3.0 94 |
17 |
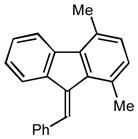 2q |
48 30 |
| 6 |
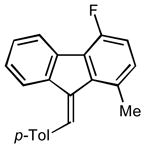 2f |
3.0 92 |
12 |
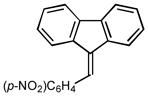 2l |
4.0 89 |
18 |
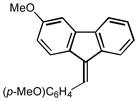 2r |
6.0 86 |
Reaction conditions: 0.5 mmol of 1, 0.025 mmol of Pd(OAc)2, 0.035 mmol of d-i-Prpf, 1ml of toluene, 120°C.
Isolated yields.
