Table 3.
Optimization of conditions for arylative cyclization of 1a.
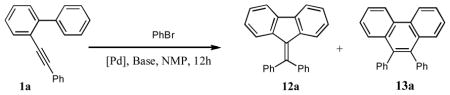 | ||||||
|---|---|---|---|---|---|---|
| # | [Pd] source | Ligand | Base | Temperature | 12a:13a Ratioa | Yield of 12a, %b |
| 1 | Pd(OAc)2 | dppp | KOAc | 120 | - | 0 |
| 2 | Pd(OAc)2 | dppe | KOAc | 120 | - | 0 |
| 3 | Pd(OAc)2 | Ph2P(CH2)5PPh2 | KOAc | 120 | - | 0 |
| 4 | Pd(OAc)2 | dppf | KOAc | 100 | 90:10 | 10 |
| 5 | Pd(OAc)2 | d-t-Bupf | KOAc | 100 | 90:10 | 12 |
| 6 | Pd(OAc)2 | d-i-Prpf | KOAc | 120 | 95:5 | 12 |
| 7 | Pd(OAc)2 | d-i-Prpf | Cs2CO3 | 120 | - | 0 |
| 8 | Pd(OAc)2 | d-i-Prpf | Et3N | 120 | 99:1 | 45 |
| 9 | Pd(OAc)2 | d-i-Prpf | EtN(i-Pr)2 | 120 | 100:0 | 60 |
| 10 | PdCl2 | d-i-Prpf | EtN(i-Pr)2 | 120 | 100:0 | 31 |
| 11 | Pd(dba)2 | d-i-Prpf | EtN(i-Pr)2 | 120 | 100:0 | 20 |
| 12 | PdCl2(CH3CN)2 | d-i-Prpf | EtN(i-Pr)2 | 120 | 100:0 | 30 |
| 13 | Pd(OAc)2 | d-i-Prpf | Bu4NBr | 120 | 100:0 | 20 |
| 14 | Pd(OAc)2 | d-i-Prpf | DABCO | 100 | 100:0 | 70c |
| 15 | Pd(OAc)2 | dppf | DABCO | 110 | 100:0 | 81 |
| 16 | Pd(OAc)2 | d-t-Bupf | DABCO | 110 | 100:0 | 80 |
| 17 | Pd(OAc)2 | d-i-Prpf | DABCO | 110 | 100:0 | 95 |
| 18 | Pd(OAc)2 | d-i-Prpf | DABCO | 120 | 100:0 | 89d |
Product ratios determined by GC/MS analysis.
Yield determined by GC/MS analysis.
Yield after 24 hrs.
Isolated yield after 6 hrs.
