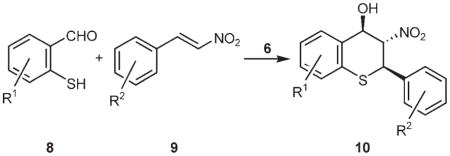
Table 2.
Enantioselective tandem Michael–Henry reaction of 2-mercaptobenzaldehydes (8) with various β-nitrostyrenes (9).a
 | |||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | 10, Yield [%]b | drc,d | ee [%]d,e |
| 1 | H | H | a, 95 (50) | 70:30 (94:6) | 86 (>99) |
| 2 | H | 2-Br | b, 97 (60) | 77:23 (93:7) | 85 (>99) |
| 3 | H | 4-Br | c, 95 (50) | 74:26 (92:8) | 76 (99) |
| 4 | H | 3-Cl | d, 96 (42) | 65:35 (92:8) | 78 (>99) |
| 5 | H | 4-Cl | e, 97 (46) | 73:27 (96:4) | 72 (97) |
| 6 | H | 2-NO2 | f, 95 (51) | 75:25 (99:1) | 78 (90) |
| 7 | H | 2-OMe | g, 94 (52) | 78:22 (93:7) | 80 (>99) |
| 8 | H | 4-OMe | h, 96 (51) | 68:32 (93:7) | 85 (>99) |
| 9 | H | 4-Me | i, 94 (48) | 71:29 (92:8) | 80 (>99) |
| 10 | 4-OMe | H | j, 95 (50) | 70:30 (91:9) | 82 (98) |
| 11 | 4-OMe | 4-Br | k, 96 (62) | 72:28 (94:6) | 80 (85) |
| 12 | 4-Me | H | l, 84 (29) | 70:30 (87:13) | 72 (95) |
| 13 | 4-Cl | H | m, 85 (32) | 70:30 (93:7) | 75 (97) |
Unless otherwise specified, all reactions were conducted at −10°C with the substituted β-nitrostyrene (0.2 mmol), the substituted 2-mercaptobenzaldehyde (0.22 mmol) and cupreine (6, 2 mol%) in anhydrous diethyl ether (10 mL) for 5 min. The absolute configuration of the major isomer 10b was determined by X-ray crystallography and the rest were assigned on the basis of the reaction mechanism.
Yields of nonseparable diastereomeric mixture after chromatography; values in parentheses are those after a single recrystallization.
Determined by 1H NMR analyses on the crude products or the recrystallized products.
Values in parantheses are those of the recrystallized products.
Values of enantiomeric excess of the major isomers as determined by HPLC analyses on a Chiralpak AD-H column (entries 1–12) or a Chiralcel OD-H column (entry 13).
