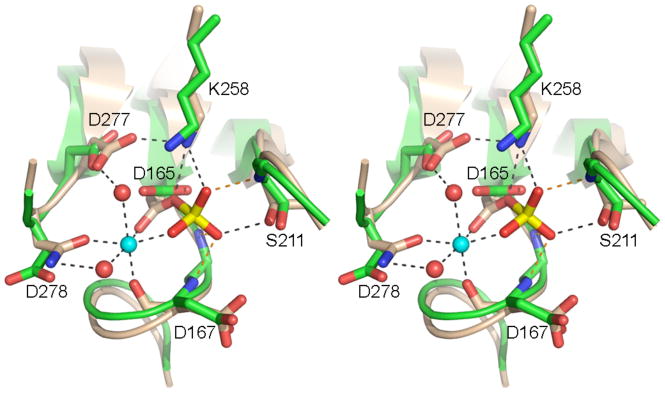
Fig. 5. Active site similarity between Pnkp phosphatase and CTD serine phosphatase.
Stereo view of the phosphatase active site from Pnkp protomer D (green) superimposed on the BeF3-modified active site of CTD phosphatase Scp1 (beige). The aspartyl-BeF3 adduct is depicted with the beryllium in yellow and the fluorines colored red (reflecting their mimicry of the phosphate of the aspartyl-phosphate intermediate). The Mg2+ of Scp1 is depicted as a cyan sphere and the associated waters as red spheres. Atomic interactions in the Scp1 active site are indicated by dashed lines. Pnkp side chains are labeled.