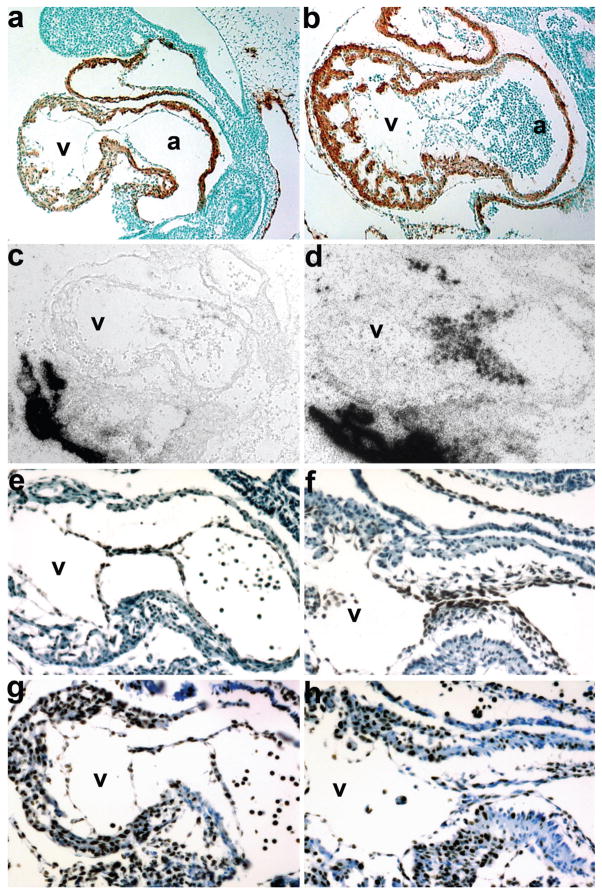
FIG. 4.
Molecular marker analysis of E10 Smad7Cre;R26DTA hypoplastic endocardial cushion phenotype. (a,b) Sagittal sections through Smad7Cre;R26DTA (a) and control littermate (b) hearts probed with α-SMA and counterstained with methyl green. Note that the cardiomyocytes within the OFT, ventricle and atria are unaffected; but that the endocardial cushions are absent in ablated mutant hearts. (c,d) Radioactive in situ hybridization detection of Periostin mRNA in genetically ablated mutant (c) and control (c) embryos reveals very few Periostin-expressing cells within the mutant cushions (c). But note robust expression within the mutant umbilical artery and embryonicmaternal connections, which is comparable with control expression levels. (e,f) High power views of mutant (e) and control (f) hearts counterstained for Nfatc1 protein. Note that even though the mutant cushions are largely devoid of endocardial cushion cells, Nfatc1 is still expressed within the intact mutant endothelium (e). As expected, unaffected control littermates exhibit both endothelial and cushion Nfatc1 expression (f). (g,h) High power views of mutant (g) and control (h) hearts stained for Ki67 protein to assess proliferation. Note the mutant cushion intact endothelial cells and myocardium exhibit comparable proliferation rates as observed in littermate controls (h).