Table 1.
Common HNO Donors Used in Biology
| Donor | Chemical Structure |
Notes |
|---|---|---|
| Angeli's salt | 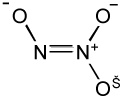 |
- Fast release of HNO |
| - Nitrite is also formed | ||
| Piloty's acid | 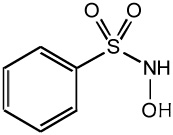 |
- Releases HNO only at high pH |
| Acyloxo nitroso "Blue compounds" | 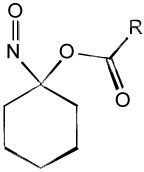 |
- Releases HNO upon cleavage of ester bond |
| - Rate of HNO release is controled by R substituent | ||
| - Reacts with thiols | ||
| IPA/NO | 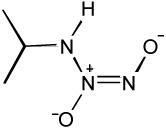 |
- Primary amine "NONOate" |
| - NO donor at lower pH | ||
| hetero-Diels-Alder cycloadduct | 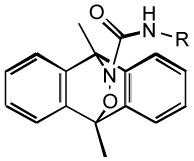 |
- Releases HNO upon photo activation |
| - Requires UV -A radiation (330 –380 nm) |
