Summary
The isthmic organizer and its key effector molecule, fibroblast growth factor 8 (Fgf8), have been cornerstones in studies of how organizing centers differentially pattern tissues. Studies have implicated different levels of Fgf8 signaling from the mid/hindbrain boundary (isthmus) as being responsible for induction of different structures within the tectal-isthmo-cerebellum region. However, the role of Fgf8 signaling for different durations in patterning tissues has not been studied. To address this, we conditionally ablated Fgf8 in the isthmus and uncovered that prolonged expression of Fgf8 is required for the structures found progressively closer to the isthmus to form. We found that cell death cannot be the main factor accounting for the loss of brain structures near the isthmus, and instead demonstrate that tissue transformation underlies the observed phenotypes. We suggest that the remaining Fgf8 and Fgf17 signaling in our temporal Fgf8 conditional mutants is sufficient to ensure survival of most midbrain/hindbrain cells near the isthmus. One crucial role for sustained Fgf8 function is in repressing Otx2 in the hindbrain, thereby allowing the isthmus and cerebellum to form. A second requirement for sustained Fgf8 signaling is to induce formation of a posterior tectum. Finally, Fgf8 is also required to maintain the borders of expression of a number of key genes involved in tectal-isthmo-cerebellum development. Thus, the duration as well as the strength of Fgf8 signaling is key to patterning of the mid/hindbrain region. By extrapolation, the length of Fgf8 expression could be crucial to Fgf8 function in other embryonic organizers.
Keywords: Fibroblast growth factors, Fgf17, Inferior colliculus, Mice
INTRODUCTION
A fundamental question in developmental biology is how distinct structures are induced by a single organizing center. Since organ development occurs over time and within the changing confines of the three dimensions of space, every model must take into consideration both the length of time a cell is exposed to organizer molecules and their respective concentration. Chick transplantation studies demonstrated two decades ago that the midbrain/hindbrain boundary region (referred to as the isthmus) is a key organizing center for the midbrain as well as the cerebellum which develops from the anterior hindbrain (rhombomere 1, r1) (Joyner et al., 2000; Simeone, 2000; Nakamura, 2001; Martinez, 2001; Wurst and Bally-Cuif, 2001; Rhinn and Brand, 2001). The dorsal midbrain forms an optic tectum in fish, amphibians and birds, whereas in mammals the tectum forms an anterior superior colliculus (SC) and posterior inferior colliculus (IC) with distinct morphologies, cytoarchitecture and sensory functions (visual vs auditory processing). The cerebellum that is induced posterior to the isthmus has a homogeneous layered cytoarchitecture in most vertebrates, but a foliated structure in birds and mammals. The isthmus is a thin epithelium that links the tectum and cerebellum dorsally. The ventral midbrain and r1, by contrast, form a continuous tegmentum with distinct foci of neurons. Given the distinct structures that form in the tectal-isthmo-cerebellum region, the isthmic organizer provides an excellent model system for addressing how organizing centers function.
Fgf8 (fibroblast growth factor 8) is considered the key isthmic organizer molecule as it is both required for development of the tectum and cerebellum (Chi et al., 2003) and is sufficient to induce both structures in gain-of-function (GOF) studies (Crossley et al., 1996; Martinez et al., 1999; Shamim et al., 1999). One aspect of the explanation for how Fgf8 can induce different structures is likely to relate to differing strengths of intracellular signaling (Nakamura et al., 2008; Sato et al., 2004). Among the various Fgf8 protein isoforms, two are abundant in the isthmus (Blunt et al., 1997; Sato et al., 2001) and have distinct properties. Fgf8b binds the Fgf receptors (FGFRs) with higher affinity (Olsen et al., 2006), and can induce a higher level of phosphorylation of ERK than Fgf8a (Sato and Nakamura, 2004). In mis-expression studies, Fgf8a induces midbrain development, whereas Fgf8b induces cerebellum tissue (Lee et al., 1997; Liu et al., 1999; Sato et al., 2001; Liu et al., 2003). Finally, both isoforms are required, as mice lacking the four Fgf8b-like splice forms have a milder phenotype than Fgf8-null mice (Guo and Li, 2007).
Another consideration in studying the isthmic organizer is that two other Fgf8 gene family members, Fgf17 and Fgf18, are expressed in broader domains than Fgf8 around the isthmus, and they have Fgf8a-like activity in gain-of-function studies (Liu et al., 2003). Since neither gene is maintained in Fgf8 conditional knock outs (CKOs), and single mutants show mild (Fgf17) or no (Fgf18) tectum/cerebellum phenotype (Xu et al., 2000; Liu et al., 2002), their contributions to isthmic organizer activity are not clear. The secreted factor Wnt1 is also expressed near the isthmus and required for midbrain and cerebellum development, but, in GOF experiments, Wnt1 does not mimic organizer activity (Matsunaga et al., 2002; Prakash et al., 2006). Thus, Fgf8 is the only secreted factor identified to date with the full spectrum of isthmic organizer activity.
If the strength of the Fgf8 signal determines the fate of midbrain/r1 cells, then lowering the amount of Fgf8 should result in fate changes consistent with Fgf8-induced structures normally found at a distance from the isthmus (low Fgf8). Indeed, unlike conditional mutants lacking Fgf8 in the isthmus (Fgf8flox/-; En1Cre/+) that have no midbrain or r1-derived tissues (Chi et al., 2003), Fgf8 hypomorphs (Fgf8neo/neo) only develop SC and lateral cerebellum structures dorsally (Chi et al., 2003), which are tissues derived from the anterior midbrain and posterior r1, respectively (Sgaier et al., 2005). Spry2-GOF; Fgf8+/- mutants that mis-express the Fgf antagonist Spry2 in the midbrain/r1 (Basson et al., 2008) and Fgfr1flox/-; En1Cre/+ conditional mutants have similar phenotypes (Trokovic et al., 2003). Furthermore, in Fgf17-/-; Fgf8+/- mutants, less of the IC and cerebellum are lost (Xu et al., 2000). In Fgf8flox/-; En1Cre/+ conditional mutants, massive cell death has been shown to accompany the early tissue loss; however, in Spry2-GOF; Fgf8+/- and Fgfr1flox/flox; En1Cre/+ mutants, this does not seem to be the case, suggesting a low level of Fgf8/17/18 signaling may be sufficient for cell survival (Chi et al., 2003; Basson et al., 2008; Trokovic et al., 2003). The results of these studies have been interpreted to mean that different levels of Fgf signaling regulate formation of different regions of the isthmo-tectal-cerebellum region, with the highest levels being required near the isthmus.
Another explanation for the mechanism of Fgf8 organizer activity would be for different parts of the tectal-isthmo-cerebellum region to require Fgf8 (and/or Fgf17/18) signaling for different lengths of time. Given that Fgf8 is initially expressed throughout most of the dorsal r1 and the isthmus and then becomes restricted to the isthmus, it is possible that Fgf8 is only transiently required to induce posterior r1 development (lateral cerebellum). In this study, we demonstrate that more sustained Fgf8 activity is progressively required for development of structures near the isthmus. We further show that the mechanism underlying the structural changes primarily involves Fgf8 regulating key developmental genes, rather than cell survival. Moreover, Fgf17 and not Fgf18 expression is maintained when Fgf8 is temporally ablated. Thus, we have uncovered that not only the strength but also the duration of Fgf8 signaling is crucial for distinguishing the distinct structures that form along the anterior-posterior axis of the tectal-isthmo-cerebellum region in the mouse.
MATERIALS AND METHODS
Temporal inactivation of Fgf8 in the mid/hindbrain
Fgf8flox/-; En2CreER/+ embryos/mice were generated by breeding Fgf8+/-; En2CreER/CreER male mice with Fgf8flox/flox females. Noon on the day a vaginal plug was detected was designated as embryonic day 0.5 (E0.5). Tamoxifen (TM; Sigma T5648) was dissolved in corn oil (Sigma C-8267) at a final concentration of 20 mg/ml. 5 mg of TM per 40g of body weight was administered at noon on the days indicated. Fgf8flox/+; En2CreER/+ and Fgf8+/-; En2CreER/+ embryos/mice were used as controls. Genotyping was performed as previously described (Meyers et al., 1998; Sgaier et al., 2005).
Histological analysis
Embryos or E18.5 brains were immersion fixed in 4% paraformaldehyde and then embedded in paraffin and sectioned at 7 μm. Adult P21 mice were perfused and the brain fixed overnight in 4% paraformaldehyde, and then 40 μm vibratome sections were stained with Hematoxylin and Eosin.
Immunohistochemistry
Immunohistochemistry was performed on 12-14 μm frozen sections using standard procedures (see http://www.mskcc.org/mskcc/html/77387.cfm). The following primary antibodies were used: anti-neurogranin (1:500, Millipore), anti-TH (1:500, Chemicon) and anti-5-HT (1:500, Immunostar). Donkey anti-rabbit IgG-Alexa488 (1:500, Molecular Probes) or donkey anti-goat IgG-Alexa555 (1:500, Molecular Probes) were used as secondary antibodies. Brains of E18.5 embryos or P21 adults were fixed with 4% paraformaldehyde at 4°C overnight and submerged sequentially in 15% and 30% sucrose/PBS, then embedded in optimal cutting temperature (OCT) compound (Sakura).
In situ hybridization
The protocol for RNA in situ hybridization is available at the Joyner lab web site (http://www.mskcc.org/mskcc/html/77387.cfm). cDNAs for Fgf8 (Crossley and Martin, 1995), Fgf8 exon 3 (Lewandoski et al., 2000), Fgf17 (Xu et al., 1999), Fgf18 (Maruoka et al., 1998), Spry1 (Minowada et al., 1999), Otx2 (Ang et al., 1994) and Wnt1 (Parr et al., 1993) were used to synthesize antisense riboprobes. Embryos were fixed in 4% paraformaldehyde/PBS, embedded in paraffin and sectioned at 6 μm. Recombination efficiency was assessed by in situ hybridization using RNA probes that detect Fgf8 exon 3, which is deleted by Cre-mediated recombination, and compared with expression of the mutant allele using the entire Fgf8 coding sequence (see Fig. 4).
Fig. 4.
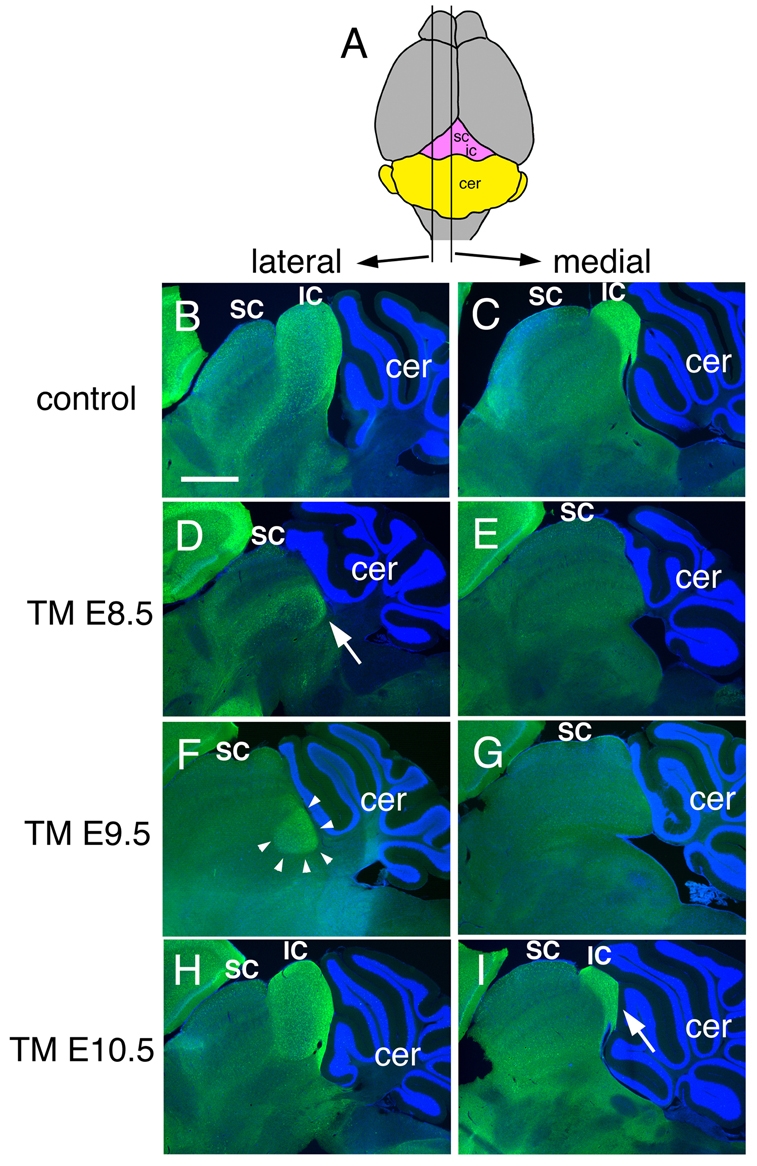
Neurogranin staining of adult brain sections shows that the inferior colliculus requires sustained Fgf8 signaling. (A) Schematic of dorsal view of an adult brain to show the level of the lateral and medial sections shown below. (B-I) Neurogranin staining of the inferior colliculus of adult Fgf8 temporal CKO embryos treated with TM at the time-points indicated. The control is an Fgf8flox/+; En2CreER/+ embryo treated with TM at E8.5. The arrow in D indicates the faint and abnormally positioned neurogranin staining in the Fgf8-E8.5 CKO. Arrowheads in F indicate the abnormal neurogranin staining in the lateral IC of a Fgf8-E9.5 CKO mutant. Arrow in I indicates the smaller medial IC in Fgf8-E10.5 CKO mice. Abbreviations are as in Fig. 1. Scale bar: 500 μm.
Assay for cell death
To detect cell death, embryos were stained with LysoTrackerT (Molecular Probes L-7528) as described previously (Grieshammer et al., 2005; Basson et al., 2008).
RESULTS
Fgf8 is required during distinct temporal windows for development of different structures in the tectal-isthmo-cerebellum region
In order to uncover whether Fgf8 is required for different lengths of time between E8.5 and E13 when it is expressed in the isthmus, we utilized an inducible Cre recombinase system to ablate Fgf8. We combined our En2 knock-in allele (Sgaier et al., 2005) that expresses CreERT2 protein (Feil et al., 1996; 1997) with a conditional floxed allele of Fgf8 (Fgf8flox) (Meyers et al., 1998). Importantly, En2CreER is expressed from E8.5 onwards across the mid/hindbrain junction in a domain that encompasses Fgf8 expression in the isthmus. Fgf8flox/-; En2CreER/+ mice were administered TM at E8.5, E9.5 or E10.5, and are referred to as Fgf8-E8.5, -E9.5 or -E10.5 CKOs. Two types of controls were analyzed, Fgf8flox/+; En2CreER/+ littermates administered TM, and Fgf8-/+; En2CreER/+ animals not administered TM (Fig. 1; also see Fig. S1M,N in the supplementary material). As CreER protein translocates into the nucleus within 6-12 hours of TM treatment, Fgf8 function should be ablated within 24-48 hours (Danielian et al., 1998; Robinson et al., 1991; Zervas et al., 2004). Strikingly, unlike Fgf8flox/-; En1Cre/+ mice that die at birth and lack the midbrain and r1, Fgf8-E8.5, -E9.5 and -E10.5 CKOs survived to adulthood. However, the mutants had a progressively greater loss of tectal-isthmo-cerebellum tissue as Fgf8 was inactivated at earlier stages (Fig. 1; also see Fig. S1 in the supplementary material; see details below).
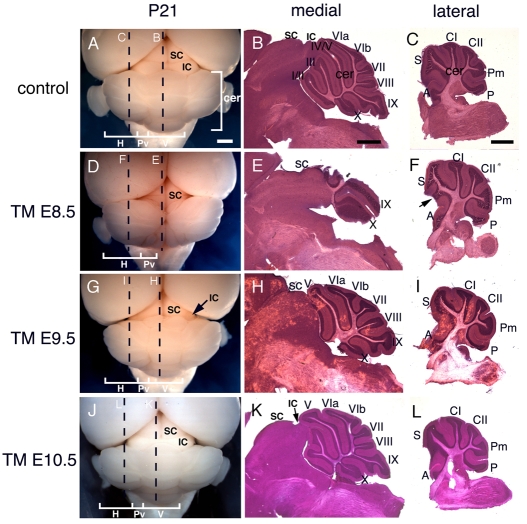
Fig. 1.
Adult brain morphology shows that Fgf8 is required for different lengths of time to form tectal-isthmo-cerebellum substructures. Dorsal view of wholemount adult brains (A,D,G,J), and Hematoxylin and Eosin-stained paramedial (B,E,H,K) and lateral (C,F,I,L) sections of Fgf8 temporal CKO embryos treated with TM at the time points indicated. The control is an Fgf8flox/+; En2CreER/+ embryo treated with TM at E8.5. Arrow in F points to bulge in simplex of Fgf8-E8.5 CKOs. Arrow in G points to loss of IC in Fgf8-E9.5 CKOs. Arrow in K points to decreased size of the IC in Fgf8-E10.5 CKOs. Dashed lines in wholemounts indicate the level of the indicated sections. A, anterior; cer, cerebellum; CI, crus I; CII, crus II; H, hemisphere; IC, inferior colliculus; I-X, vermis lobules, hemisphere lobules; S, simplex; SC, superior colliculus; P, copula pyramidis; Pm, paramedianus; Pv, paravermis; V, vermis. Scale bars: 1 mm.
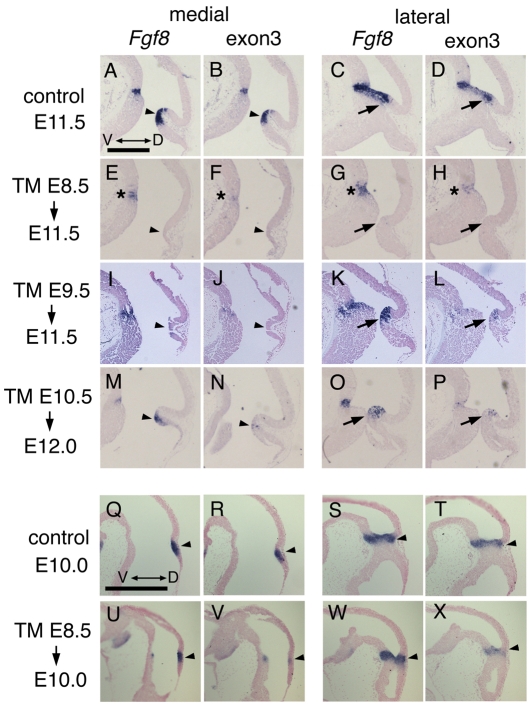
To determine the extent of recombination with the three TM induction time-points, we performed RNA in situ analysis with a probe that detects the Fgf8 exon 3 sequences flanked by loxP sites in the conditional allele (Lewandoski et al., 2000). The three Fgf8 temporal CKOs were analyzed at E11.5 or E12 when recombination should be complete. Analysis at this stage also allowed us to assess whether a phenotype could be detected in the mutants soon after loss of Fgf8 function. An Fgf8 probe containing the entire cDNA was used to assess whether the endogenous Fgf8 gene continued to be expressed after Fgf8 function was ablated. As previously reported (Lewandoski et al., 2000) in control embryos (Fgf8flox/+; En2CreER/+), the expression pattern detected with the exon 3 probe was the same as that with the full-length Fgf8 probe (Fig. 2A-D). In E11.5 Fgf8-E8.5 CKOs (3/3), no expression of Fgf8 was detected in the dorsal isthmus with either probe (Fig. 2E-H). Furthermore, most of the dorsal isthmo-cerebellum region was deleted in these mutants, which could account for the loss of Fgf8 expression. The phenotype nevertheless demonstrated effective knock-out of Fgf8 in these mutants before 3 days post-TM. Consistent with the milder adult phenotype of Fgf8-E9.5 CKOs compared with Fgf8-E8.5 CKOs, in three out of four E11.5 Fgf8-E9.5 CKOs there was a reduction of tissue in the dorsal isthmus region, but less than that in Fgf8-E8.5 CKOs (see Fig. S2 in the supplementary material for variability of recombination efficiency). Importantly, in Fgf8-E9.5 CKOs, no expression of Fgf8 was detected with the two Fgf8 probes in the dorsal midline, indicating effective ablation of Fgf8. Faint Fgf8 exon 3 expression was detected in some dorsal-lateral sections with the exon 3 probe and stronger expression was observed with the full length probe (Fig. 2I-L). In E12 Fgf8-E10.5 CKOs (36 hours after TM administration), a very small number of cells were detected with the exon 3 probe, although the vast majority of cells detected with the full-length Fgf8 probe did not express exon 3 (3/3; compare Fig. 2N,P with M,O). Moreover, the cerebellar primordium was clearly reduced in size in Fgf8-E10.5 CKOs, but less so than in Fgf8-E9.5 CKOs (Fig. 2M). In the ventral mid/hindbrain border region of all the Fgf8 temporal CKOs, weak expression of Fgf8 was detected with the exon 3 probe and stronger expression was observed with the full-length probe, especially more laterally, indicating less efficient recombination ventrally (Fig. 2E-H, asterisks).
Fig. 2.
Recombination is efficient by 36 hours following TM in Fgf8 temporal CKOs. (A-X) RNA in situ hybridization with the two Fgf8 probes indicated on adjacent medial or lateral sections of Fgf8 temporal CKO embryos treated with TM at the time-points indicated. The ages of the embryos analyzed are indicated under the arrows at the left. The control embryo is Fgf8flox/+; En2CreER/+ treated with TM at E8.5. The transcripts detected with the Fgf8 and exon 3 probes were greatly diminished in the dorsomedial isthmus (arrowheads). (K,L,O,P) Although the Fgf8 mutant transcript was detected in the dorsolateral isthmus of Fgf8-E9.5 CKO and Fgf8-E10.5 CKOs, the normal transcript was greatly diminished (arrows). (E-H, asterisks) The normal Fgf8 transcript was only partially reduced ventrally. (N,P,V,X, arrowheads) The normal Fgf8 transcript was greatly diminished by 36 hours after TM administration in the dorsal isthmus. Scale bars: in A, 500 μm for A-P; in Q, 500 μm for Q-X.
To assess when Fgf8 expression is extinguished in the Fgf8 temporal CKOs, Fgf8-E8.5 CKO embryos were assessed 24 and 36 hours after TM was administered. Expression of Fgf8 exon 3 was detected at 24 hours, although it appeared slightly reduced compared with controls (data not shown). By 36 hours after TM treatment, Fgf8 exon 3 expression was greatly reduced (Fig. 2Q-X), but, as with the Fgf8-E10.5 CKOs analyzed at E12, faint expression remained dorsally. In addition, a morphological phenotype was apparent as a reduction in the depth of the isthmus flexure (under arrowhead). Taken together, the gene expression results and timing of the onset of a phenotype (see Fig. S5 in the supplementary material, abnormal Otx2 expression) demonstrate that our Fgf8 temporal CKO scheme results in a decrease in Fgf8 expression by 24 hours post-TM, with a functional decrease in Fgf8 by 36 hours. Loss of Fgf8 is then complete in the dorsal isthmus by 48 hours and the degree of recombination is similar with the three TM treatments.
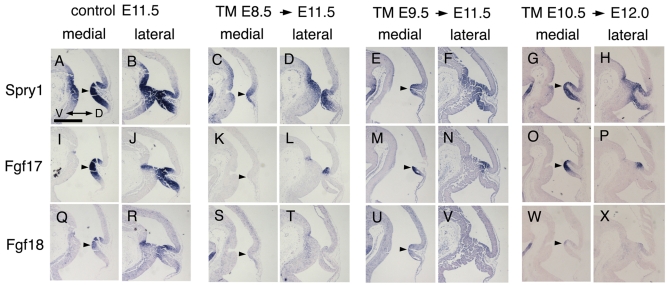
To assess the degree to which Fgf signaling was decreased in the temporal Fgf8 CKOs, we analyzed expression of the Fgf8 direct target gene, Spry1 (Minowada et al., 1999; Liu et al., 2003), at E11.5 or E12 (Fig. 3). Spry1 expression was greatly reduced in the dorsal mid/hindbrain regions of all three Fgf8 temporal CKOs (n=3 for each genotype; Fig. 3A-H). The low level of Fgf signaling remaining in the mutants could be stimulated by Fgf8 released from the few remaining wild-type (WT) lateral and ventral cells and/or signaling from the related proteins Fgf17 and Fgf18 (Minowada et al., 1999). In Fgf8 CKOs using En1-Cre, expression of Fgf17 and Fgf18 was found to be lost by approximately the 13- or 7-somite stage, respectively (Chi et al., 2003). Interestingly, although clearly reduced, Fgf17 expression was maintained in the dorsal mid/hindbrain region of the three Fgf8 CKOs (Fig. 3I-X), whereas Fgf18 expression was barely detectable in all three mutants. These results demonstrate that Fgf8/17/18 signaling was greatly reduced in the three Fgf8 temporal CKOs, but a low level of signaling remained. Importantly, based on Spry1 expression, a similar level of signaling was present in the remaining mid/hindbrain tissue in the three Fgf8 temporal CKO mutants, demonstrating that any differences in dorsal phenotypes in tectal-isthmo-cerebellum structures observed were due to different requirements for sustained Fgf8 signaling in specific structures.
Fig. 3.
Loss of Fgf18 expression and reduction of Fgf17 and Sprty1 expression soon after Fgf8 is ablated. (A-X) RNA in situ hybridization with the probes indicated of adjacent medial or lateral sections in each column of Fgf8 temporal CKO embryos treated with TM at the time-points indicated. The ages of the embryos analyzed are indicated to the right of the arrows at the top. The control is an Fgf8flox/+; En2CreER/+ embryo treated with TM at E8.5. Arrowheads indicate the expressions of Spry1 (A,C,E,G), Fgf17 (I,K,M,O) and Fgf18 (Q,S,U,W) in the dorsal-medial part of caudal midbrain-isthmus-r1 region. Scale bar: 500 μm.
Fgf8 is required only transiently after E8.5 for development of the superior colliculus and lateral cerebellum
We first examined the degree to which transient expression of Fgf8 in the isthmus from E8.5 to ∼E10 is sufficient to support development of tectal-isthmo-cerebellum structures by administering TM to Fgf8flox/-; En2CreER/+ embryos at E8.5, as Fgf8 function is ablated at approximately E10. In wholemount and sections of the adult brain, Fgf8-E8.5 CKO mice showed either a major (3/5) or partial (2/5) deletion of the medial cerebellum (vermis) compared with that of controls, as well as an absence of the inferior colliculus (IC) in all mutants (Fig. 1A,B,D,E; also see Fig. S1A-E in the supplementary material). Based on tracing the remaining lobules on a series of sections from the vermis to the hemispheres (data not shown), and, given that only lobules VI and VII are contiguous with the four major lobules of the hemispheres and that VIII/IX extend more laterally than I-V, we suggest that the major lobules remaining in the three severe mutants correspond to lobules IX and X (Larsell, 1952). The two other mutants had a severe reduction of the anterior lobules I-V and a reduction of lobule VIII. In contrast to the medial cerebellum, the lateral cerebellum (hemispheres) appeared similar to WT, except that the simplex lobule had a small anterior bulge in all mutants (5/5; Fig. 1F, arrow). Curiously, the most anterior cerebellar tissue of all mutants was abnormally fused to the SC in the medial one-third of the mutant cerebellum (Fig. 1E, also see Fig. S1A′-E′ in the supplementary material). The tissue had a fully formed layered cytoarchitecture but the white matter (inner layer) abutted the tectum rather than the outer molecular layer (see Fig. S1 in the supplementary material, asterisks).
In order to characterize the loss of the IC further, we performed immnohistochemistry to detect the IC marker neurogranin on a series of sections along the medial-lateral axis. Whereas neurogranin marked the entire medial IC of control postnatal day 21 (P21) mice in all sections (Fig. 4B,C), in Fgf8-E8.5 CKOs there were no neurogranin-positive cells in the medial midbrain (Fig. 4E), and only a small number of neurogranin-expressing cells were detected laterally that were abnormally positioned ventrally (Fig. 4D). These data show that the medial IC does not form and that the lateral IC develops abnormally when Fgf8 is only transiently expressed at E8.5.
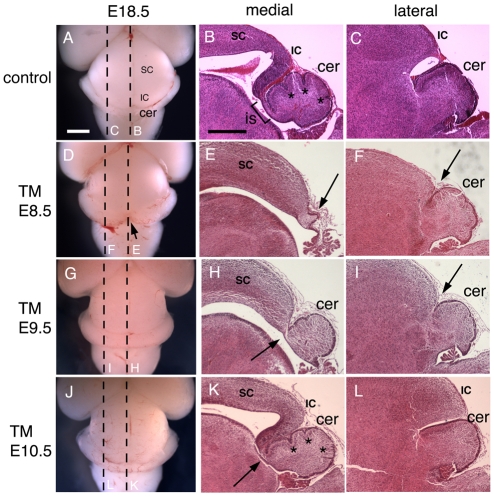
We next examined E18.5 embryonic brains (Fig. 5A-F), a stage when the isthmus is clearly visible on sections. As in the P21 Fgf8-E8.5 CKOs, at E18.5 on medial sagittal sections of mutants, the IC was absent (Fig. 5B,E) and only a tiny cerebellum-like structure was present, fused to the caudal tip of the remaining SC (Fig. 5E, arrow). Thus, no isthmus was present in Fgf8-E8.5 CKOs. In lateral sections, the cerebellum appeared similar to WT in morphology (Fig. 5C,F), whereas the midbrain was reduced in size (Fig. 5F, arrow). At E18.5 neurogranin was expressed only in the lateral IC (see Fig. S3A-C,C′ in the supplementary material). In lateral sections of Fgf8-E8.5 CKOs, neurogranin was detected in fewer cells than normal and was abnormally positioned ventrally (see Fig. S3D-F in the supplementary material), a phenotype similar to that seen at P21.
Fig. 5.
E18.5 brain morphology shows that Fgf8 is required for different lengths of time to form tectal-isthmo-cerebellum substructures. Dorsal view of wholemount adult brains (A,D,G,J), and Hematoxylin and Eosin-stained paramedial (medial; B,E,H,K) and lateral (C,F,I,L) sections of Fgf8 temporal CKO embryos treated with TM at the time-points indicated. The control is an Fgf8flox/+; En2CreER/+ embryo. Dashed lines in wholemounts indicate the level of the indicated sections. Arrows in D and E indicate loss of the vermis, in addition to the inferior colliculus in Fgf8-E8.5 CKO mutants, and in F the shallow isthmus flexure. The arrow in H indicates the lack of isthmus and inferior colliculus in Fgf8-E9.5 CKO mutants, and in I the shallow isthmus flexure. The arrow in K indicates that the isthmus is missing in Fgf8-E10.5 CKO mutants, in addition to the cerebellum fissures being shallower than normal (B,F, asterisks). Abbreviations are as in Fig. 1. Scale bars: in A, 1 mm for left column; in B, 500 μm for right two columns.
Although recombination was less efficient ventrally, we examined the differentiation of midbrain dopaminergic neurons by assessing tyrosine hydroxylase (TH) expression and hindbrain serotonergic neurons by assessing 5-hydroxy tryptophan (5-HT) (Brodski et al., 2003; Zervas et al., 2004; Ye et al., 1998; Blaess et al., 2006). Unlike the loss of tissue in the dorsal midbrain/r1 of Fgf8-E8.5 CKOs at E18.5, there was no obvious difference in the number of TH-positive neurons of the substantia nigra (SN) and ventral tegmental area (VTA) between control and Fgf8-E8.5 CKO embryos (see Fig. S4A,B in the supplementary material; data not shown). However, the TH+ neurons of the locus ceruleus (LoC) in the dorsal r1 of Fgf8-E8.5 CKO embryos had an abnormal distribution, being more spread out than normal (see Fig. S4B in the supplementary material, arrow). In only one of three mutants, there appeared to be a slight decrease in the number of 5-HT-positive neurons in the ventral r1 (see Fig. S4C,D in the supplementary material).
Fgf8 is required after ∼E11 for development of the medial inferior colliculus and anterior-medial cerebellum
We next examined the degree to which Fgf8 continues to be required after ∼E11 for development of the vermis and IC. Strikingly, in contrast to adult Fgf8-E8.5 CKOs, medial sections of Fgf8-E9.5 CKO cerebellum showed that only the anterior lobules (I-V) of the vermis were missing or greatly reduced in size (n=4; Fig. 1H, also see Fig. S1F-I in the supplementary material). In three of the mutants, lobules I-III were replaced by cerebellum tissue that was abnormally fused to the midbrain, as in the milder Fgf8-E8.5 CKOs (Fig. 1H; also see Fig. S1F-H in the supplementary material). In the fourth mutant, an additional small lobule was present anterior to lobule IV/V (see Fig. S1I in the supplementary material, arrow). In addition, in two mutants, the base of lobe VIII was shifted slightly posteriorly (Fig. 1H; also see Fig. S1F-I in the supplementary material). As expected, the hemispheres appeared normal (Fig. 1I). In contrast to the cerebellum, the medial IC of Fgf8-E9.5 CKO mice was greatly reduced, based on morphology (Fig. 1G,H) and an absence of neurogranin staining in the medial sections (Fig. 4G). In the lateral midbrain, the swellings of the IC were discernible, although smaller than normal (Fig. 1G, arrow), and the domain of neurogranin staining was shifted ventrally and greatly reduced, although less so than in Fgf8-E8.5 CKOs (Fig. 4F). Thus, more of the vermis and lateral IC formed when Fgf8 function was extended from ∼E10 to ∼E11 (difference in phenotypes obtained in Fgf8-E8.5 CKOs compared with Fgf8-E9.5 CKOs), but the medial IC was severely compromised.
Analysis of morphological changes in the brains of E18.5 Fgf8-E9.5 CKOs showed defects consistent with the defects seen in adult mutants (Fig. 5G-I). The IC and isthmus were absent (Fig. 5H, arrow), and the medial cerebellum was present but was smaller than normal and possessed fewer folia. In lateral sections of Fgf8-E9.5 CKOs, the constriction between the IC and cerebellum was more shallow than normal (Fig. 5I, arrow). Neurogranin expression was only slightly reduced compared with WTs in the most lateral tectum, whereas, on progressively more medial sections, the neurogranin staining was progressively reduced and some cells were displaced ventrally (see Fig. S3A-C,G-I in the supplementary material).
Fgf8 is required after ∼E12 for development of the isthmus and to form a complete medial inferior colliculus and anterior-medial cerebellum
Finally, we examined the degree to which Fgf8 continues to be required after ∼E12 for development of the anterior vermis and medial IC. Unlike Fgf8-E9.5 CKOs, the IC of adult and E18.5 Fgf8-E10.5 CKOs was only slightly smaller than in controls (Fig. 1J-L, Fig. 5J-L). Neurogranin staining confirmed that the IC was present in medial and lateral sections and that the medial IC was only slightly smaller than normal (Fig. 4H,I; also see Fig. S3J-L in the supplementary material). Furthermore, only the anterior-most lobules (I-III) of the cerebellum were partially (2/3) or fully fused (1/3) and reduced in size (Fig. 1K,L; also see Fig. S1J-L in the supplementary material). By contrast, as in the other Fgf8 temporal mutants, the most anterior region of the medial vermis contained cerebellum tissue fused to the tectum (3/3) and lobule VIII was shifted slightly posteriorly (2/3; Fig. 1K; also see Fig. S1J in the supplementary material). Furthermore, the isthmus was absent in all Fgf8-E10.5 CKO embryos at E18.5 (Fig. 5K, arrow). As two of the Fgf8-E10.5 CKOs had the same number of lobules as littermate controls, this suggested that the abnormal anterior cerebellum tissue fused to the tectum is ectopic tissue, possibly caused by transformation of isthmus tissue into cerebellum tissue. A much milder version of this phenotype was seen in only one control animal (0/6 Fgf8+/flox; En2CreER/+; 1/3 Fgf8+/-; En2CreER/+; see Fig. S1N in the supplementary material).
Taken together, our series of temporal Fgf8 CKOs demonstrated that sustained Fgf8 signaling was progressively required to maintain development of structures centered around the isthmus.
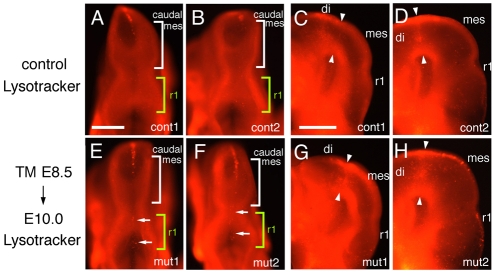
The number of apoptotic cells increases only slightly in the caudal midbrain, isthmus and dorsal r1 when Fgf8 is ablated by E10
One possible reason for the loss of tissue in the three Fgf8 temporal CKO mutants is that cell survival was compromised. In Fgf8flox/-; En1Cre/+ CKOs, Cre is active at E8.5 and massive cell death was observed using Lysotracker (labels acidic organelles in apoptotic cells; Grieshammer et al., 2005) in the midbrain and anterior hindbrain from E8.75 to E9.50 (Chi et al., 2003). We therefore analyzed apoptosis in Fgf8-E8.5 CKOs (the mutants with the greatest tissue loss) soon after Fgf8 function was ablated (E10). Unlike Fgf8 CKOs using En1-Cre, in the r1 of Fgf8-E8.5 CKOs (n=6), there was only a slight increase in the number of apoptotic cells in the dorsal midline at E10 (Fig. 6A,B,E,F). In the midbrain of control embryos, many apoptotic cells were detected in the anterior dorsal midline (Fig. 6A,B). In E10 mutants, the dorsal midline strip of apoptotic cells extended more posteriorly than in controls (Fig. 6E,F). Curiously, in lateral regions of the anterior midbrain (primordium of the SC in normal embryos), there was an increase in the number of apoptotic cells of E10 embryos (Fig. 6C,D,G,H). To determine whether cell death is increased in Fgf8 CKOs at a later stage, we analyzed E10.5 embryos (n=7). However, no difference in apoptosis could be detected between the CKOs and WT controls (data not shown). These results suggest that cell death is not likely to be responsible for the severe loss of the IC, isthmus and vermis in Fgf8-E8.5 CKOs.
Fig. 6.
Apoptosis is not greatly increased near the dorsal isthmus when Fgf8 is ablated by ∼E10. Dorsal (A,B,E,F) and lateral (C,D,G,H) views of E10 control (A-D; Fgf8flox/+; En2CreER/+, TM at E8.5) and Fgf8-E8.5 CKO embryos (E-H) stained with Lysotracker. Arrows in E and F indicate a slight increase in apoptotic cells in the dorsal midline of the r1 in mutants. Arrowheads in G and H indicate an increase in apoptosis in the diencephalon/mesencephalic border region of mutants. di, diencephalons; mes, mesencephalon; r1, rhombomere1.
Otx2 and Wnt1 are expanded posteriorly and Gbx2 is decreased in the absence of Fgf8 function
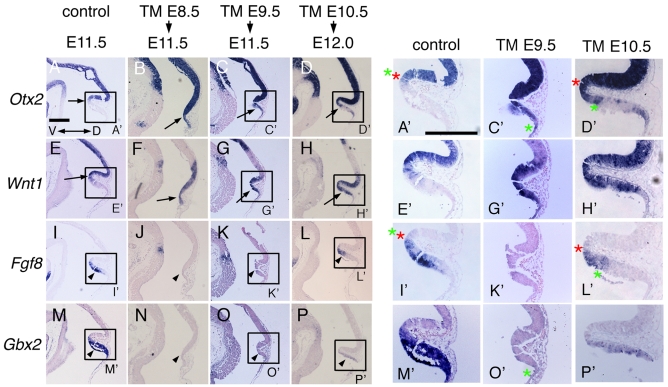
We next examined whether differential changes in target gene expression could account for the successively milder phenotypes seen in Fgf8 temporal CKOs as TM was given later. The expression of Otx2, Wnt1, Gbx2 and the Fgf8 mutant transcript was examined, as Otx2 and Wnt1 are normally restricted to the midbrain and downregulated in the r1 by Fgf8 signaling, and Gbx2 is restricted to the r1 and repressed by Otx2 (Joyner et al., 2000; Wurst and Bally-Cuif, 2001; Nakamura, 2001). Importantly, Otx2 is sufficient to transform r1 into midbrain tissue and Gbx2 is needed to establish the presumptive r1-3 region in mice (Wassarman et al., 1997; Broccoli et al., 1999).
At E11.5 in sagittal sections of control embryos (Fgf8flox/+; En2CreER/+, TM at E8.5), Otx2 was detected throughout the dorsal midbrain, with a posterior border at the isthmus, adjacent to the anterior border of Fgf8 (Fig. 7A,A′,I,I′; also see Fig. S5A-E″ in the supplementary material). In lateral sections, there was a slight overlap in the expression domains of the two genes. Wnt1 had a similar posterior border to that of Otx2 in control embryos but was expressed only in the posterior most midbrain and in the dorsal and ventral midlines (Fig. 7E,E′; also see Fig. S5A′-E′ in the supplementary material). Gbx2 had an anterior border adjacent to Otx2/Wnt1 similar to Fgf8 (Fig. 7M,M′; also see Fig. S5A‴-E‴ in the supplementary material). In medial sections of Fgf8-E8.5 CKO embryos, Otx2 and Wnt1 extended to the posterior tip of what remained of the tectal-isthmo-cerebellar region and Fgf8 and Gbx2 were absent (Fig. 7B,F,J,N; also see Fig. S5F,F′,G,G′ in the supplementary material). In more-lateral sections, Wnt1 was expanded posteriorly to the tip of the cerebellum, whereas Otx2 was shifted posteriorly only slightly and was absent from all but the most posterior cerebellum (see Fig. S5H-L,H′-L′ in the supplementary material). Weak expression of Gbx2 was detected only in the lateral r1 (see Fig. S5F″-L″, lateral part of K″,L″ in the supplementary material).
Fig. 7.
Otx2 and Wnt1 are expanded posteriorly and Gbx2 is diminished soon after Fgf8 is ablated. (A-P′) RNA in situ hybridization with the probes indicated of adjacent medial sections in each column of Fgf8 temporal CKO embryos treated with TM at the time-points indicated. The ages of the embryos analyzed are indicated under the arrows at the top. The control is an Fgf8flox/+; En2CreER/+ embryo treated with TM at E8.5. Rectangles indicate area shown at higher magnification to the right. Green asterisks indicate caudal limit of the Otx2 expression. Red asterisks indicate rostral limit of the Fgf8 expression. Arrows in A-H indicate that Otx2 and Wnt1 are expanded posteriorly in the dorsal isthmus-r1 of mutants. (I-P) Dorsal Fgf8 and Gbx2 expression are diminished in mutants (arrowheads). Scale bar: in A, 300 μm for A-P; in A′, 300 μm for A′-P′.
In contrast to Fgf8-E8.5 CKOs, Otx2 was expanded posteriorly in the entire dorsal cerebellum only in the most medial sections of Fgf8-E9.5 CKO embryos (Fig. 7C,C′; also see Fig. S5M in the supplementary material) in which no Fgf8 mutant transcript was detected (Fig. 7K,K′; also see Fig. S5M″ in the supplementary material). In slightly lateral sections, Otx2 was shifted only slightly posteriorly into the Fgf8 mutant transcript domain and was absent from all but the most posterior cerebellum (see Fig. S5N-Q,N″-Q″ in the supplementary material). In Fgf8-E9.5 CKO embryos, Wnt1 was expanded posteriorly in the entire dorsal isthmo-cerebellum more laterally than Otx2, but not as laterally as in Fgf8-E8.5 CKO embryos (Fig. 7G,G′; also see Fig. S5M′-Q′ in the supplementary material). The Gbx2-negative area in the isthmo-cerebellum region of Fgf8-E9.5 CKO embryos was smaller than in Fgf8-E8.5 CKO embryos (Fig. 7O,O′; also see Fig. S5M‴-O‴ in the supplementary material), although Gbx2 expression appeared weaker than in controls in the lateral regions (see Fig. S5P‴,Q‴ in the supplementary material).
Correlating with the mildest phenotype in Fgf8-E10.5 CKO embryos, Otx2 was not expanded homogeneously to the tip of the cerebellum even in the most medial sections at E12 and instead was expanded posteriorly only into the isthmus overlapping with the Fgf8 mutant transcript, with scattered ectopic expression in the most posterior cerebellum (Fig. 7D,D′,L,L′; also see Fig. S6A,A″,F,F″ in the supplementary material). Dorsolaterally, Otx2 was only expanded slightly posteriorly and the amount of overlap with the Fgf8 mutant transcript diminished progressively laterally (see Fig. S6B-E,B″-E″,G-J,G″-J″ in the supplementary material). Wnt1 expression was expanded posteriorly to the tip of the cerebellum only in the most medial sections (Fig. 7H,H′; also see Fig. S6A′,F′ in the supplementary material), and, in more lateral sections, the caudal limit of Wnt1 was extended only slightly posteriorly (diminishing progressively laterally) and was also expressed at the caudal tip of the cerebellum (Fig. 7H,H′; and compare Fig. S6A′-E′ with F′-J′ in the supplementary material). Finally, Gbx2 expression was much weaker than in controls only in the most medial sections (Fig. 7P; and compare Fig. S6A‴-E‴ with F‴-J‴ in the supplementary material).
In summary, in Fgf8 temporal CKO embryos, Otx2 and Wnt1 expression was expanded posteriorly into the isthmus and cerebellum, and Gbx2 expression was correspondingly diminished. Moreover, there was a correlation between the degree to which the embryonic expression domains were altered and the degree of loss of medial cerebellum tissue in each of the adult mutants.
DISCUSSION
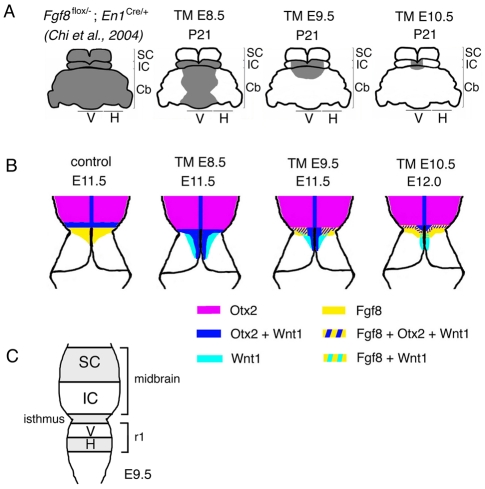
By conducting temporal conditional ablation of Fgf8 in the dorsal isthmus, we have uncovered that Fgf8 signaling from the isthmus is required for different lengths of time for development of different tectal-isthmo-cerebellum structures. Importantly, the tectal-isthmo-cerebellum structures that develop from precursors increasingly further away from the isthmus at midgestation require Fgf8 for gradually less time (Fig. 8A,C). When Fgf8 was ablated at ∼E10 (Fgf8-E8.5 CKO), morphological and gene expression changes in the isthmus were immediately apparent. When Fgf8 was instead ablated at ∼E12 (Fgf8-E10.5 CKO) the isthmus, anterior-medial cerebellum and part of the medial IC nevertheless did not form. Thus, sustained Fgf8 function is required to form the structures near the isthmus. Therefore, the cells that receive the highest levels of Fgf8 signaling appear to also require Fgf8 signaling for the longest duration to develop properly. Together, these findings suggest that (1) the SC and lateral cerebellum require Fgf8 only for a brief period during E8.5-E10, (2) the posterior vermis and lateral IC require Fgf8 during E8.5-E11, and (3) the isthmus, medial-anterior cerebellum and part of the medial IC require Fgf8 during the entire time that Fgf8 is expressed (E8.5-E12.5).
Fig. 8.
Schematic showing the progressive loss of structures and more severe changes in gene expression near the isthmus as Fgf8 is ablated earlier. (A) Back view of adult brain showing cerebellum [medial vermis (V) and lateral hemispheres (H)] and tectum (IC and SC). Shading indicates the regions of the tectum and cerebellum that are lost when Fgf8 is conditionally ablated from the isthmus at the times indicated or at E8.5 in Fgf8flox-; En1Cre embryos (Chi et al., 2004). The isthmus is lost in all the mutants (not shown). (B) Changes in gene expression seen at the times indicated in Fgf8 temporal CKO embryos. Fgf8 indicates the mutant transcript. (C) Dorsal view of E9.5 neural tube showing the primordial for the SC, IC, isthmus, vermis (V) and hemispheres (H).
A key question is why the IC, isthmus and anterior-vermis are dependent on Fgf8 for a protracted length of time. Unlike Fgf8 CKOs using En1-Cre (E8.5 ablation), in which cell death appears to largely account for loss of the entire tectal-isthmo-cerebellum region (Chi et al., 2003), in Fgf8-E8.5 CKOs (ablation at E10), only a small increase in cell death was observed near the isthmus at E10. Interestingly, in all three Fgf8 temporal CKOs, Fgf18 expression was lost in the dorsal mid/hindbrain region, whereas Fgf17 expression was maintained, although reduced. We suggest that the low level of Fgf8/17 signaling that remains in the temporal Fgf8 CKOs is sufficient to ensure survival of most midbrain/r1 cells near the isthmus. Indeed, the main increase in cell death was seen at the farthest distance from the isthmus (anterior midbrain) where Fgf signaling was not detected based on Spry1 expression. Thus, cell death can account for only a minor part of the loss of brain structures.
If impaired cell survival does not account for loss of IC, isthmus and vermis tissue in Fgf8 temporal CKOs, then what does? In terms of the isthmus and vermis (derived from the anterior r1), we propose that these regions are transformed into a midbrain fate owing to mis-expression of Otx2. Strikingly, our study showed that the degree of posterior expansion of Otx2 into the dorsal r1 correlates with the degree of loss of the medial cerebellum in the three different temporal Fgf8 CKOs (Fig. 8A,B). Although Wnt1 also was expanded posteriorly in Fgf8 temporal mutants, it is not likely to be responsible for the loss of cerebellum for several reasons. First, the mis-expression of Otx2 correlates better with the cerebellum phenotype, as Wnt1 was expanded much more extensively into the r1 even in Fgf8-E10.5 CKOs that form most of the vermis. In addition, Wnt1 mis-expression in the r1 does not cause a transformation into midbrain (Matsunaga et al., 2002; Prakash et al., 2006), whereas Otx2 is required for tectum development (Acampora et al., 1995; Matsuo et al., 1995; Ang et al., 1996; Martinez-Barbera et al., 2001) and is capable of transforming the dorsal r1 into tectum (Millet et al., 1999; Katahira et al., 2000). The reduction in Gbx2 expression in Fgf8 temporal CKOs is also not likely to account for the loss of the vermis because, in Gbx2 CKO mutants (Gbx2flox/; En1Cre/+), a cerebellum still develops (Li et al., 2002). Supporting the idea that mis-expression of Otx2 in the r1 of temporal Fgf8 CKOs is involved in the loss of medial cerebellum tissue, knockdown of otx2 in zebrafish fgf8 mutants (ace) allows differentiation of some cerebellum cell types (Foucher et al., 2006). Thus, our studies have uncovered a crucial role of sustained isthmic Fgf8 signaling in repressing Otx2 in the isthmus and the r1, thereby creating an Otx2-negative region in which the isthmus and medial cerebellum can form.
The cause of the tectum phenotype seems to be distinct from that of the isthmo-cerebellum phenotype. In the temporal Fgf8 CKOs, there was a clear increase in cell death in the anterior midbrain, unlike in the r1 where little increased apoptosis was observed. Curiously, the anterior midbrain is the primordium of the SC and a SC nevertheless forms in all three Fgf8 temporal CKOs. A similar phenotype was seen in Spry2-GOF; Fgf8+/- mutants (Basson et al., 2008) where it was proposed that the cell death in the anterior midbrain was accompanied by a transformation of the remaining posterior tectum (the primordium of the IC) into SC owing to lowered Fgf signaling. We suggest that a similar mechanism underlies the Fgf8 temporal CKO tectum phenotype. However, unlike in Spry2-GOF; Fgf8+/- mutants, in Fgf8 temporal CKOs, the IC, isthmus and vermis are transformed into a SC by the remaining Fgf17 signaling and the SC precursors die owing to a lack of Fgf signaling. Thus, a second requirement for Fgf8 signaling throughout E8.5-E13 is to induce formation of a complete IC.
Our studies demonstrate a sustained requirement for Fgf8 signaling, even after E12, in maintaining the posterior gene expression borders of Otx2 and Wnt1. It is possible that Fgf8 inhibits Otx2 and Wnt1 expression, as ectopic Fgf8b leads to their repression (Martinez at al., 1999; Liu et al., 1999; Sato et al., 2001; Liu and Joyner, 2001; Liu et al., 2003), and reduction of Fgf8 signaling in chick embryonic r1 or in zebrafish ace mutants that lack only the isthmo-cerebellum region causes otx2 to expand posteriorly (Jaszai et al., 2003; Suzuki-Hirano et al., 2005). Our study and others have found that Gbx2 expression is reduced when Fgf8 signaling is decreased (Chi et al., 2003; Rhinn et al., 2003; Trokovic et al., 2003; Basson et al., 2008; Saarimäki-Vire et al., 2007). Since mis-expression of Otx2 in the r1 leads to repression of Gbx2 (Millet et al., 1999; Katahira et al., 2000), one possibility was that the ectopic Otx2 in the r1 represses Gbx2. However, in our Fgf8 temporal CKOs, Gbx2 expression was not complementary to Otx2. Thus, Fgf8 plays a third sustained role in maintaining the isthmus expression borders of a number of key genes involved in tectal-isthmo-cerebellum development.
Curiously, in other mouse mutants with reduced Fgf signaling that lack part of the vermis and do not have massive cell death, caudal expansion of Otx2 into the r1 was not detected (Fgf17-/-, Fgf17-/-, Fgf8+/-, Fgf18-/-, Fgf8neo/neo, Fgfr1flox/flox, En1Cre/+, Spry2-GOF, Fgf8+/-) (Xu et al., 2000; Liu et al., 2002; Ohbayashi et al., 2002; Chi et al., 2003; Trokovic et al., 2003; Basson et al., 2008). Therefore, the mechanism by which the medial cerebellum is deleted in these mutants appears to be different from that of the Fgf8 temporal CKOs. As Fgf17 (and probably Fgf18) cannot repress Otx2 expression (Liu et al., 2003), in these mutants the remaining Fgf8 activity might be sufficient to repress Otx2. Basson et al. hypothesized that expansion of the roof plate in the r1 is the primary cause of loss of the vermis in Spry2-GOF; Fgf8+/- embryos (Basson et al., 2008). As Spry inhibits pathways other than Fgf, this could account for a different mechanism in these mutants compared with that of temporal Fgf8 CKOs. Of possible evolutionary significance, the phenotype of the Fgf8-E8.5 CKOs most closely resembles zebrafish ace mutants (Jaszai et al., 2003), although the tectum is larger in the zebrafish mutants and it is not known whether an anterior transformation occurs. The results of our study thus raise the question of whether another Fgf functions early to maintain cell survival in ace mutants.
In summary, our study taken together with previous studies, suggest the following explanation for how a single isthmic organizer can induce distinct brain structures: during development of the dorsal tectal-isthmo-cerebellum region in mouse, the highest level and longest length of Fgf8 signaling is required for the isthmus to form. Progressively lower levels of signaling and shorter durations of signaling are required for development of tectal structures from posterior to anterior, and for regions of the cerebellum from medial to lateral (Fig. 8C). It is now important to elucidate the additional contributions of distinct Fgf8 isoforms to patterning. Fgf8 also is required throughout E8.5 to E13 to regulate gene expression borders in the isthmus, and a sufficient level of Fgf signaling is required to maintain cell viability. Thus, the duration of Fgf8 expression, as well as the strength of Fgf8 signaling, are both crucial factors for the specification of distinct structures within the tectal-isthmo-cerebellum region.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/21/3617/DC1
Supplementary Material
We are grateful to Drs Praveen Raju and Sandra Wilson for helpful discussions and comments on the manuscript. We thank Dr Gail Martin for providing the Fgf8 mutant alleles, and Drs D. Ornitz, M. Frohman, B. Hogan, J. Rossant, A. MacMahon, and Martin for providing RNA in situ probes. T.S. was supported by grants from Toyobo Biotechnology Foundation, Uehara Memorial Foundation and Japan Society for the Promotion of Science, and A.L.J. by grant R01 HD050767-01 from the NIH. Deposited in PMC for release after 12 months.
References
- Acampora, D., Mazan, S., Lallemand, Y., Avantaggiato, V., Maury, M., Simeone, A. and Brûlet, P. (1995). Forebrain and midbrain regions are deleted in Otx2-/- mutants due to a defective anterior neuroectoderm specification during gastrulation. Development 121, 3279-3290. [DOI] [PubMed] [Google Scholar]
- Ang, S. L., Conlon, R. A., Jin, O. and Rossant, J. (1994). Positive and negative signals from mesoderm regulate the expression of mouse Otx2 in ectoderm explants. Development 120, 2979-2989. [DOI] [PubMed] [Google Scholar]
- Ang, S. L., Jin, O., Rhinn, M., Daigle, N., Stevenson, L. and Rossant, J. (1996). A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development 122, 243-252. [DOI] [PubMed] [Google Scholar]
- Basson, A. M., Echevarria, D., Ahn, C. P., Sudarov, A., Joyner, A. L., Mason, I. J., Martinez, S. and Martin, G. R. (2008). Specific regions within the embryonic midbrain and cerebellum require different levels of FGF signaling during development. Development 135, 889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaess, S., Corrales, J. D. and Joyner, A. L. (2006). Sonic hedgehog regulates Gli activator and repressor functions with spatial and temporal precision in the mid/hindbrain region. Development 133, 1799-1809. [DOI] [PubMed] [Google Scholar]
- Blunt, A. G., Lawshe, A., Cunningham, M. L., Seto, M. L., Ornitz, D. M. and MacArthur, C. A. (1997). Overlapping expression and redundant activation of mesenchymal fibroblast growth factor (FGF) receptors by alternatively spliced FGF-8 ligands. J. Biol. Chem. 272, 3733-3738. [DOI] [PubMed] [Google Scholar]
- Broccoli, V., Boncinelli, E. and Wurst, W. (1999). The caudal limit of Otx2 expression positions the isthmic organizer. Nature 401, 164-168. [DOI] [PubMed] [Google Scholar]
- Brodski, C., Weisenhorn, D. M., Signore, M., Sillaber, I., Oesterheld, M., Broccoli, V., Acampora, D., Simeone, A. and Wurst, W. (2003). Location and size of dopaminergic and serotonergic cell populations are controlled by the position of the midbrain-hindbrain organizer. J. Neurosci. 23, 4199-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, C., Martinez, S., Wurst, W. and Martin, G. R. (2003). The isthmicorganizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development 130, 2633-2644. [DOI] [PubMed] [Google Scholar]
- Crossley, P. H. and Martin, G. R. (1995). The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121, 439-451. [DOI] [PubMed] [Google Scholar]
- Crossley, P. H., Martinez, S. and Martin, G. R. (1996). Midbrain development induced by FGF8 in the chick embryo. Nature 380, 66-68. [DOI] [PubMed] [Google Scholar]
- Danielian, P. S., Muccino, D., Rowitch, D. H., Michael, S. K. and McMahon, A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323-1326. [DOI] [PubMed] [Google Scholar]
- Feil, R., Brocard, J., Mascrez, B., LeMeur, M., Metzger, D. and Chambon, P. (1996). Ligand-activated site-specific recombination in mice. Proc. Natl. Acad. Sci. USA 93, 10887-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil, R., Wagner, J., Metzger, D. and Chambon, P. (1997). Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem. Biophys. Res. Commun. 237, 752-757. [DOI] [PubMed] [Google Scholar]
- Foucher, I., Mione, M., Simeone, A., Acampora, D., Bally-Cuif, L. and Houart, C. (2006). Differentiation of cerebellar cell identities in absence of Fgf signalling in zebrafish Otx morphants. Development 133, 1891-1900. [DOI] [PubMed] [Google Scholar]
- Grieshammer, U., Cebrián, C., Ilagan, R., Meyers, E., Herzlinger, D. and Martin, G. R. (2005). FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132, 3847-3857. [DOI] [PubMed] [Google Scholar]
- Guo, Q. and Li, J. Y. (2007). Distinct functions of the major Fgf8 spliceform, Fgf8b, before and during mouse gastrulation. Development 134, 2251-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaszai, J., Reifers, F., Picker, A., Langenberg, T. and Brand, M. (2003). Isthmus-to-midbrain transformation in the absence of midbrain-hindbrain organizer activity. Development 130, 6611-6623. [DOI] [PubMed] [Google Scholar]
- Joyner, A. L., Liu, A. and Millet, S. (2000). Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr. Opin. Cell Biol. 12, 736-741. [DOI] [PubMed] [Google Scholar]
- Katahira, T., Sato, T., Sugiyama, S., Okafuji, T., Araki, I., Funahashi, J. and Nakamura, H. (2000). Interaction between Otx2 and Gbx2 defines the organizing center for the optic tectum. Mech. Dev. 91, 43-52. [DOI] [PubMed] [Google Scholar]
- Larsell, O. (1952). The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J. Comp. Neurol. 97, 281-356. [DOI] [PubMed] [Google Scholar]
- Lee, S. M., Danielian, P. S., Fritzsch, B. and McMahon, A. P. (1997). Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development 124, 959-969. [DOI] [PubMed] [Google Scholar]
- Lewandoski, M., Sun, X. and Martin, G. R. (2000). Fgf8 signaling from the AER is essential for normal limb development. Nat. Genet. 26, 460-463. [DOI] [PubMed] [Google Scholar]
- Li, J., Lao, Z. and Joyner, A. L. (2002). Changing requirements for gbx2 in development of the cerebellum and maintenance of the mid/hindbrain organizer. Neuron 36, 31-43. [DOI] [PubMed] [Google Scholar]
- Liu, A. and Joyner, A. L. (2001). EN and GBX2 play essential roles downstream of FGF8 in patterning the mouse mid/hindbrain region. Development 128, 181-191. [DOI] [PubMed] [Google Scholar]
- Liu, A., Losos, K. and Joyner, A. L. (1999). FGF8 can activate Gbx2 and transform regions of the rostral mouse brain into a hindbrain fate. Development 126, 4827-4838. [DOI] [PubMed] [Google Scholar]
- Liu, Z., Xu, J., Colvin, J. S. and Ornitz, D. M. (2002). Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev. 16, 859-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, A., Li, J. Y. H., Bromleigh, C., Lao, Z., Niswander, L. A. and Joyner, A. L. (2003). FGF17 and FGF18 have different midbrain regulatory properties from FGF8b and activated FGF Receptors. Development 130, 6175-6185. [DOI] [PubMed] [Google Scholar]
- Martínez, S. (2001). The isthmic organizer and brain regionalization. Int. J. Dev. Biol. 45, 367-371. [PubMed] [Google Scholar]
- Martinez, S., Crossley, P. H., Cobos, I., Rubenstein, J. L. and Martin, G. R. (1999). FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development 126, 1189-1200. [DOI] [PubMed] [Google Scholar]
- Martinez-Barbera, J. P., Signore, M., Boyl, P. P., Puelles, E., Acampora, D., Gogoi, R., Schubert, F., Lumsden, A. and Simeone, A. (2001). Regionalisation of anterior neuroectoderm and its competence in responding to forebrain and midbrain inducing activities depend on mutual antagonism between OTX2 and GBX2. Development 128, 4789-4800. [DOI] [PubMed] [Google Scholar]
- Maruoka, Y., Ohbayashi, N., Hoshikawa, M., Itoh, N., Hogan, B. M. and Furuta, Y. (1998). Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech. Dev. 74, 175-177. [DOI] [PubMed] [Google Scholar]
- Matsunaga, E., Katahira, T. and Nakamura, H. (2002). Role of Lmx1b and Wnt1 in mesencephalon and metencephalon development. Development 129, 5269-5277. [DOI] [PubMed] [Google Scholar]
- Matsuo, I., Kuratani, S., Kimura, C., Takeda, N. and Aizawa, S. (1995). Mouse Otx2 functions in the formation and patterning of rostral head. Genes Dev. 9, 2646-2658. [DOI] [PubMed] [Google Scholar]
- Meyers, E. N., Lewandoski, M. and Martin, G. R. (1998). An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 18, 136-141. [DOI] [PubMed] [Google Scholar]
- Millet, S., Campbell, K., Epstein, D. J., Losos, K., Harris, E. and Joyner, A. L. (1999). A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature 401, 161-164. [DOI] [PubMed] [Google Scholar]
- Minowada, G., Jarvis, L. A., Chi, C. L., Neubuser, A., Sun, X., Hacohen, N., Krasnow, M. A. and Martin, G. R. (1999). Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 126, 4465-4475. [DOI] [PubMed] [Google Scholar]
- Nakamura, H. (2001). Regionalization of the optic tectum: combinations of gene expression that define the tectum. Trends Neurosci. 24, 32-39. [DOI] [PubMed] [Google Scholar]
- Nakamura, H., Sato, T. and Suzuki-Hirano, A. (2008). Isthmus organizer for mesencephalon and metencephalon. Dev. Growth Differ. 50, 113-118. [DOI] [PubMed] [Google Scholar]
- Ohbayashi, N., Shibayama, M., Kurotaki, Y., Imanishi, M., Fujimori, T., Itoh, N. and Takada, S. (2002). FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 16, 870-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, S. K., Li, J. Y. H., Bromleigh, C., Eliseenkova, A. V., Ibrahimi, O., Lao, Z., Zhang, F., Linhardt, R. J., Joyner, A. L. and Mohammadi, M. (2006). Structural basis by which alternative splicing modulates the organizer activity of FGF8 in the brain. Genes Dev. 20, 185-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr, B. A., Shea, M. J., Vassileva, G. and McMahon, A. P. (1993). Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 119, 247-261. [DOI] [PubMed] [Google Scholar]
- Prakash, N., Brodski, C., Naserke, T., Puelles, E., Gogoi, R., Hall, A., Panhuysen, M., Echevarria, D., Sussel, L., Weisenhorn et al. (2006). A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development 133, 89-98. [DOI] [PubMed] [Google Scholar]
- Rhinn, M. and Brand, M. (2001). The midbrain-hindbrain boundary organizer. Curr. Opin. Neurobiol. 11, 34-42. [DOI] [PubMed] [Google Scholar]
- Rhinn, M., Lun, K., Amores, A., Yan, Y. L., Postlethwait, J. H. and Brand, M. (2003). Cloning, expression and relationship of zebrafish gbx1 and gbx2 genes to Fgf signaling. Mech. Dev. 120, 919-936. [DOI] [PubMed] [Google Scholar]
- Robinson, S. P., Langan-Fahey, S. M., Johnson, D. A. and Jordan, V. C. (1991). Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab. Dispos. 19, 36-34. [PubMed] [Google Scholar]
- Saarimäki-Vire, J., Peltopuro, P., Lahti, L., Naserke, T., Blak, A. A., Vogt Weisenhorn, D. M., Yu, K., Ornitz, D. M., Wurst, W. and Partanen, J. (2007). Fibroblast growth factor receptors cooperate to regulate neural progenitor properties in the developing midbrain and hindbrain. J. Neurosci. 27, 8581-8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, T. and Nakamura, H. (2004). The Fgf8 signal causes cerebellar differentiation by activating Ras-ERK signaling pathway. Development 131, 4275-4285. [DOI] [PubMed] [Google Scholar]
- Sato, T., Araki, I. and Nakamura, H. (2001). Inductive signal and tissue responsiveness defining the tectum and the cerebellum. Development 128, 2461-2469. [DOI] [PubMed] [Google Scholar]
- Sato, T., Joyner, A. L. and Nakamura, H. (2004). How does Fgf signaling from the isthmic organizer induce midbrain and cerebellum development? Dev. Growth Differ. 46, 487-494. [DOI] [PubMed] [Google Scholar]
- Sgaier, S. K., Millet, S., Villanueva, M. P., Berenshteyn, F., Song, C., Joyner, A. L. (2005). Morphogenetic and cellular movements that shape the mouse cerebellum; insights from genetic fate mapping. Neuron 45, 27-40. [DOI] [PubMed] [Google Scholar]
- Shamim, H., Mahmood, R., Logan, C., Doherty, P., Lumsden, A. and Mason, I. (1999). Sequential roles for Fgf4, En1 and Fgf8 in specification and regionalisation of the midbrain. Development 126, 945-959. [DOI] [PubMed] [Google Scholar]
- Simeone, A. (2000). Positioning the isthmic organizer where Otx2 and Gbx2 meet. Trends Genet. 16, 237-240. [DOI] [PubMed] [Google Scholar]
- Suzuki-Hirano, A., Sato, T. and Nakamura, H. (2005). Regulation of isthmic Fgf8 signal by sprouty2. Development 132, 257-265. [DOI] [PubMed] [Google Scholar]
- Trokovic, R., Trokovic, N., Hernesniemi, S., Pirvola, U., Vogt Weisenhorn, D. M., Rossant, J., McMahon, A. P., Wurst, W. and Partanen, J. (2003). FGFR1 is independently required in both developing mid- and hindbrain forsustained response to isthmic signals. EMBO J. 22, 1811-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman, K. M., Lewandoski, M., Campbell, K., Joyner, A. L., Rubenstein, J. L., Martinez, S. and Martin, G. R. (1997). Specification of the anterior hindbrain and establishment of a normal mid/hindbrain organizer is dependent on Gbx2 gene function. Development 124, 2923-2934. [DOI] [PubMed] [Google Scholar]
- Wurst, W. and Bally-Cuif, L. (2001). Neural plate patterning: upstream and downstream of the isthmic organizer. Nat. Rev. Neurosci. 2, 99-108. [DOI] [PubMed] [Google Scholar]
- Xu, J., Liu, Z. and Ornitz, D. M. (2000). Temporal and spatial gradients of Fgf8 and Fgf17 regulate proliferation and differentiation of midline cerebellar structures. Development 127, 1833-1843. [DOI] [PubMed] [Google Scholar]
- Xu, J., Lawshe, A., MacArthur, C. A. and Ornitz, D. M. (1999). Genomic structure, mapping, activity and expression of fibroblast growth factor 17. Mech. Dev. 83, 165-178. [DOI] [PubMed] [Google Scholar]
- Ye, W., Shimamura, K., Rubenstein, J. L., Hynes, M. A. and Rosenthal, A. (1998). FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93, 755-766. [DOI] [PubMed] [Google Scholar]
- Zervas, M., Millet, S., Ahn, S. and Joyner, A. L. (2004). Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron 43, 345-357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.