Fig. 1. Superposition of the active site residues of rat 3α-HSD (AKR1C9) and 3α,20β-HSD [11].
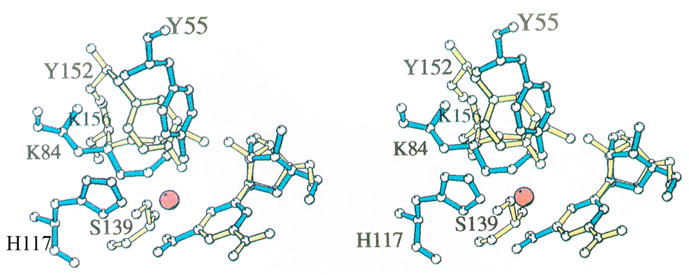
Comparison of AKR and SDR enzymes in which the protein folds differ but the active site residues superimpose. Active site residues in 3α-HSD (blue) superimposed with those in 3α,20β–HSD, a member of the SDR superfamily (yellow) [7]. All non-hydrogen atoms in Tyr 55, Lys 84, His 117, and nicotinamide ribose in the 3α-HSD-NADP+ binary complex and in Ser 139, Tyr 152, Lys 156, and nicotinamide ribose in the 3α,20β-HSD-NAD+ binary complex are shown. The water molecule in 3α-HSD that may mimic the carbonyl oxygen in a 3-ketosteroid substrate is represented by a red sphere. The superposition was based on the nicotinamide ring position, excluding the carboxamide substituent at the C3 position. The reason for excluding this substituent was that 3α,20β-HSD transfers the pro-S hydrogen, while 3α-HSD transfers the pro-R hydrogen, so that although the nicotinamide rings lie in the same plane, they are flipped 180° relative to one another and the carboxamide groups do not superimpose. This figure was prepared using MOLSCRIPT. Reproduced with permission from Biochemistry 1996 35, 10702–10711. Copyright 1996 the American Chemical Society.
