Abstract
Mu opioid receptor (MOR) regulation of somatodendritic dopamine neurotransmission in the ventral tegmental area (VTA) was investigated using conventional microdialysis in freely moving rats and mice. Reverse dialysis of the MOR agonist, DAMGO (50, 100 μM), into the VTA of rats produced a concentration-dependent increase in dialysate DA concentrations. Basal dopamine overflow in the VTA was unaltered in mice lacking the MOR gene. However, basal GABA overflow in these animals was significantly increased, while glutamate overflow was decreased. Intra-VTA perfusion of DAMGO to wildtype (WT) mice increased dopamine overflow. GABA concentrations were decreased whereas glutamate concentrations in the VTA were unaltered. Consistent with the loss of MOR, no effect of DAMGO was observed in MOR knockout (KO) mice.
These data provide the first direct demonstration of tonically active MOR systems in the VTA that regulate basal glutamatergic and GABAergic neurotransmission in this region. We hypothesize that increased GABAergic neurotransmission following constitutive deletion of MOR is due to the elimination of a tonic inhibitory influence of MOR on GABA neurons in the VTA, whereas decreased glutamatergic neurotransmission in MOR KO mice is a consequence of intensified GABA tone on glutamatergic neurons and/or terminals. As a consequence, somatodendritic dopamine release is unaltered. Furthermore, MOR KO exhibit no positive correlation between basal dopamine levels and the glutamate/GABA ratio observed in WT animals.
Together our findings indicate a critical role of VTA MOR in maintaining an intricate balance between excitatory and inhibitory inputs to dopaminergic neurons.
Keywords: Mu opioid receptor, ventral tegmental area, dopamine, glutamate, GABA, knockout mice
Introduction
Mu opioid receptors (MOR) are enriched in the ventral tegmental area (VTA), the site of origin of the mesocorticolimbic dopamine system (Mansour et al., 1988; Dilts and Kalivas, 1989; Garzon and Pickel, 2001; Svingos et al., 2001). This dopamine system is implicated in mediating the reinforcing effects of natural rewards and drugs of abuse (Bozarth and Wise, 1986; Schultz, 1997; Wise, 2002).
Several lines of evidence suggest that the VTA is a critical site mediating the rewarding effects of MOR agonists. Thus, intra-VTA microinjections of MOPr agonists elicit conditioned place preference CPP (Phillips and LePiane, 1980; Bals-Kubik et al., 1993) and animals will work to obtain infusions of MOR agonists into this region (Devine and Wise, 1994; David and Cazala, 1994). Therefore, alteration of MOR function in the VTA can have a profound effect on drug induced CPP and self-administration.
Microdialysis studies demonstrated that acute intracerebroventricular (Spanagel et al., 1990a; Spanagel et al., 1990b), intra-VTA (Devine et al., 1993) or systemic (Rada et al., 1991) administration of MOR agonists increase dopamine overflow in the nucleus accumbens (NAc). This action is thought to contribute, at least in part, to the reinforcing effects of these agents. Morphological data suggest that MOR are primarily located on non-dopaminergic neurons in the VTA (Mansour et al., 1987; Mansour et al., 1988). Intracellular recordings in slice preparations of the VTA revealed that morphine increases the firing rate of dopamine neurons but inhibits the firing rate of non-dopaminergic neurons (Johnson and North, 1992b). Although the identity of the non-dopaminergic neurons was not definitively determined, these findings led to the hypothesis that activation of MOR on GABA neurons inhibits their activity, thereby, decreasing GABA release and disinhibiting VTA DA neurons. As a consequence DA release in the NAc and medial prefrontal cortex (mPFC) is increased. However, the data upon which this hypothesis is based were obtained in a slice preparation in which connectivity of functional circuits is not preserved. Fundamental question exist as to whether MOR activation affects GABA release in awake animals.
Alterations in glutamate transmission are recognized to play an important role in shaping the pattern of DA neuronal activity in the brain. Only two studies have examined opioid regulation of glutamate transmission in the VTA (Johnson et al., 1992; Manzoni and Williams, 1999). In these, slice preparations from halothane or isoflurane anesthetized animals were used. Importantly, however, general anesthetics may affect impulse activity, as well as basal glutamate (and GABA) transmission (Shiraishi et al., 1997; Liachenko et al., 1999). To date, studies examining MOR regulation of VTA glutamate transmission in the awake animal are lacking. Furthermore, no studies have simultaneously evaluated the influence of MOR agonists on GABA, glutamate and dopamine transmission in the VTA Similarly, the role of endogenous MOR systems in regulating glutamate and GABA transmission in the VTA is unknown. The present studies used in-vivo microdialysis in the awake animal to address these issues.
Materials and methods
Male Sprague-Dawley rats (Charles River Laboratories, USA; 300–350g), wild-type (WT) and MOPr knockout (KO) mice (CDTA, Orleans, France; 30–35g) were housed in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care and experiments conformed to guidelines of the NIH/NIDA Intramural Research Program Institutional Care and Use Committee. The generation of mice lacking MOPr has been described previously (Matthes et al., 1996; Filliol et al., 2000). Breeding pairs of homozygous knockout mice, maintained on a pure C57BL/6 genetic background, were obtained from hybrid mutant mice (129 SVJ-C57BL/6 backgrounds) by backcrossing over 15 generations.
Rats were anesthetized with Equithesin (sodium pentobarbital, chloral hydrate, and magnesium sulphate; 9.72 mg/ml; 3 ml/kg, i.p) and stereotaxically implanted with a unilateral microdialysis guide cannulae (CMA/11, CMA/Microdialysis, Acton, MA, USA) in the VTA (from Bregma in mm: AP - 5.8–6.0, ML ± 0.6, V - 7.7, according to the atlas of Paxinos and Watson (1998). Mice were anesthetized with a combination of Ketamine (80 mg/kg; i.p.) and Xylazine (8 mg/kg, i.p). Standard stereotaxic procedures were used to implant unilateral microdialysis guide cannulae (CMA/7, CMA Microdialysis, Acton, MA, USA) in the VTA (from Bregma in mm: AP - 3.0, ML ± 0.4–0.5, V – 4.1, according to the atlas of Paxinos and Franklin (2001). After surgery animals were single housed in the colony room and allowed to recover for 5 days prior to commencement of the experiments.
Microdialysis experiments were conducted as previously described (Chefer et al., 2005). Microdialysis probes (CMA/11, 0.24×1mm membrane for rats and CMA/7, 0.24×1 mm membrane for mice) were manually inserted into the microdialysis cannulae ~15 hr before experiments and the animals were placed into a Plexiglas test chamber (26 × 26 × 33 cm). Prior to measurements, each probe was flushed overnight (0.3 μL/min) with artificial cerebrospinal fluid (aCSF) containing (in mM): 145 NaCl, 2.8 KCl, 1.2 MgCl2, 1.2 CaCl2, 0.25 ascorbic acid, and 5.4 d-glucose, adjusted to pH 7.2 using NaOH or H3PO4 (HPLC grade). During the experiment, fresh aCSF was perfused at 1 μL/min. After a 60 min equilibration period, 10 min dialysate sample collection commenced. All drugs were administered into the VTA by reverse dialysis. In order to validate the identity of glutamate and GABA as measured by CE-LF technique in our experiments, two groups of animals were perfused with the glutamate uptake inhibitor L-trans-pyrrolidine-2,4-dicarboxylic acid (tPDC, 1 mM) or the GABA uptake inhibitor 1-(2-(((Diphenylmethylene)imino)oxy)ethyl)-1,2,5,6-tetrahydro-3-pyridine-carboxylic acid (NO-711, 10 μM). For these experiments 3 × 10 min consecutive samples were collected to quantify basal neurotransmitter overflow. The aCSF was then changed to that containing the inhibitors. After a 30 min equilibration period, three consecutive samples were collected.
For studies assessing the effects of the MOR agonist DAMGO, 3 consecutive baseline samples were collected. The aCSF was then changed to that containing 50 or 100 μM of DAMGO. The concentrations of DAMGO used were based on previous microdialysis studies of the effects of opioids on extracellular dopamine levels in the rat CNS (Spanagel et al., 1990b). After a 30 min equilibration period, three consecutive 10 min samples were collected. The perfusion solution was then changed to regular aCSF and three additional baseline samples were collected following a 30 min equilibration period.
Importantly, animals were tethered in the microdialysis chambers ca. 16 hrs prior to the commencement of sample collection. As such, they were fully acclimatized to the chambers and were in a sedentary state during experiments.
Samples were analyzed for dopamine using HPLC coupled to electrochemical detection (CMA/200 refrigerated microinjector (CMA microdialysis, North Chelmsford, MA), a BAS PM-80 pump (BAS, West Lafayette, IN) and a BAS LC-4C amperometric detector). The mobile phase (0.15 M sodium phosphate, 2.24 mM sodium octanesulfonic acid, 0.94 mM EDTA and 13% methanol (vol/vol), adjusted to pH 5.0) was filtered through a 0.22 μm nylon filter and degassed by a BAS on-line degasser and pumped through the system at a flow rate of 0.47 ml/min. Dopamine was separated on a BAS C18 column (100 mm × 2.0 mm × 3 μm) and detected on a glassy carbon working electrode at an oxidation potential of +700 mV vs. Ag/AgCl. Dialysate dopamine levels were quantified by external standard curve calibration, using peak heights for quantification. The retention time for dopamine was 2.5–3.0 min and the limit of detection was below 0.25 nM.
Amino acid content was quantified using a capillary electrophoresis P/ACE™ MDQ system (Beckman, USA) coupled to an external ZETALIF laser-induced fluorescence detector (Picometrics, France). The excitation was performed by a diode laser (Picometrics, France) at a wavelength of 410 nm. Emission wavelength was 490 nm. Separations were carried out in a fused-silica capillary (50 μm ID, 350 μm OD, Polymicro Technologies, Phoenix, AZ). The capillary was 62 cm long (46 cm from injection to detection window). An automatic derivatization procedure suitable for unattended derivatization and injection of the samples by the P/ACE™ MDQ system was developed. Handling of buffers and derivatization solutions located in the buffer tray of the P/ACE™ MDQ system and hydrodynamic injections were performed by applying positive or negative pressure at the capillary inlet. Sample tubes contained 2 μl of dialysate. The capillary was flushed with 0.9 μl of H20 applying pressure in the H20 vial (volume calculated using the Expert software from Beckman). Next, the capillary was loaded with 0.020 μl of NaCN (300 mM in 0.5 M Borate buffer, pH 10.5) and 0.040 μl of NDA (15 mM in 75% DMSO). Then, 0.33 μl of the contents of the capillary (including the NaCN, the NDA and 0.27 μl of water) were delivered into the dialysate by applying negative pressure in the sample vial. A brief pressure pulse was delivered into the sample vial in order to push all the solutions to the bottom of the vial and ensure proper mixing. The capillary was then conditioned by flushing with 0.1 M NaOH (4 μl) followed by H20 (3 μl) and then filled with running buffer (3 μl). After approximately 5 minutes of derivatization, 0.015 μl of the mixture were injected into the capillary. Separation was achieved by applying a 24 kV potential at 33°C. The running buffer consisted of sodium borate buffer (75 mM, pH 9.2) including 10 mM hydroxypropyl-β-cyclodextrine and 70 mM sodium dodecyl sulfate; to which 5% methanol was added daily. Under these conditions, GABA and glutamate were resolved within 11 minutes and the limit of detection was below 1 nM for both analytes.
Dialysate dopamine, GABA and glutamate concentrations are expressed as absolute values (nM) or as a percentage from baseline levels. The effects of tPDC and NO-711 were analyzed with a repeated-measure ANOVA with two within-subjects factors: drug challenge and time. The effects of DAMGO were analyzed using a two-factor repeated-measures ANOVA, with two between-subjects factor (genotype and DAMGO concentration) and two within-subjects factors (drug challenge: basal vs. drug-evoked; and time). To evaluate the overall DAMGO-induced response, the area under the curve (AUC) value for the four samples collected after DAMGO perfusion was calculated for each animal, according to the standard trapezoid method. The formula used was AUC = [0.5(B + S1) d + 0.5(S1 + S2) d + 0.5 (S2 + S3)d+…+ 0.5 (Sn−1 + Sn)d) − (Bdn)], where B is the average of the samples collected during baseline, Sx are the values of each fraction collected during drug challenge, n is the total number of fractions collected during drug challenge, and d is the duration of each fraction (in min). The AUC values obtained and values for basal neurotransmitter levels were analyzed using a one-way ANOVA with genotype as the independent factor. A correlation (Pearson’s r) analysis was used to assess the relationships between basal dopamine levels and baseline glutamate/GABA ratio. Data are presented as mean ± SEM. The accepted value of significance was p ≤ 0.05.
Results
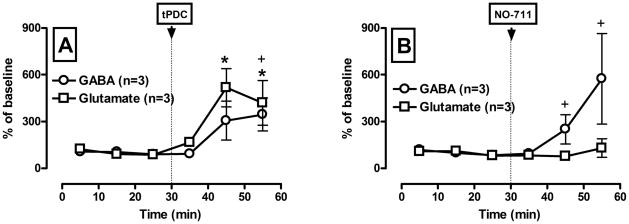
Figure 1 shows GABA and glutamate outflow in response to intra-VTA perfusion of the glutamate and GABA uptake blockers tPDC (A) and NO-711 (B), which were administered into the rats VTA via reverse microdialysis. Intra-VTA infusion of tPDC (Fig. 1, A) significantly increased dialysate glutamate and GABA levels (F(1,17) = 6.94; p < 0.01 and F(1,17) = 3.92; p = 0.03, respectively). The time course of this increase differed. Glutamate level rose during the first 10 min sample interval following tPDC infusion and a significant increase was observed during the second 10 min interval. In contrast, a significant increase in GABA overflow was first apparent during the third sample interval. The delay in the GABA response suggests that the increase in GABA overflow may be secondary to changes in glutamate levels. However, there was no neurotransmitter × drug challenge (F(1,8) = 0.98; p = 0.37) or neurotransmitter × drug challenge × time (F2,8) = 0.76; p = 0.42) interaction for tPDC. Therefore, we can not conclude that tPDC modify GABA and glutamate overflow differently. On the other hand, there was neurotransmitter × drug challenge × time interaction (F2,8) = 4.98; p = 0.04) for NO-711 (Fig. 1, B), indicating that NO-711 modify GABA and glutamate overflow in a different way. Intra-VTA infusion of NO-711 significantly increased GABA (F(1,17) = 3.4; p = 0.047), but not glutamate (F(1,17) = 0.75; p = 0.6) levels. Taken together this data confirmed the identity of glutamate and GABA as measured by CE-LIF technique in our experiments.
Figure 1.
Pharmacological validation of the separation of glutamate (A) and GABA (B) by the CE-LIFD technique employed.
Glutamate and GABA uptake inhibitors (tPDC and NO-711, respectively) were administered via reverse dialysis in rats. Microdialysis samples were collected every 10 min. Ordinate: GABA and glutamate concentration expressed as percentage (mean ± SEM) of the baseline values (n = 3 animals per group). Abscissa: time in min. * denotes a significant difference from baseline for glutamate and + denotes a significant difference from baseline for GABA (p ≤ 0.05, Tukey test).
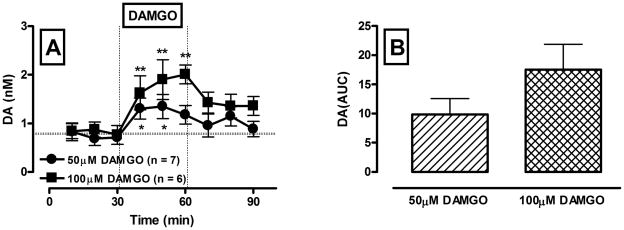
Figure 2 shows the effect of intra-VTA perfusion of the MOR agonist, DAMGO (50 and 100 μM), on dialysate levels of dopamine in rats. DAMGO produced a significant and concentration-dependent increase in dopamine levels in the VTA (drug challenge effect: F(1,11) = 112.05; p < 0.01; DAMGO concentration effect: F(1,11) = 6.66; p = 0.026 ).
Figure 2.
Concentration-dependent increases in somatodendritic dopamine overflow following reverse dialysis of the MOR agonist DAMGO into the VTA in rats.
A – Time course of dialysate dopamine levels before and following reverse dialysis of DAMGO (50 and 100 μM, black circles and black squares, respectively) into the VTA. Ordinate: dopamine concentration in nM. Data are expressed as the means ± S.E.M., n -number of animals in each experimental group (6–7 animals per group). Abscissa: time in min. Microdialysis samples were collected every 10 min. * and ** denote significant differences between basal and drug-evoked levels for two concentrations of DAMGO accordingly (p ≤ 0.05, Tukey test). B – Bar graphs of AUC values for the dopamine response to DAMGO expressed as means ± S.E.M.
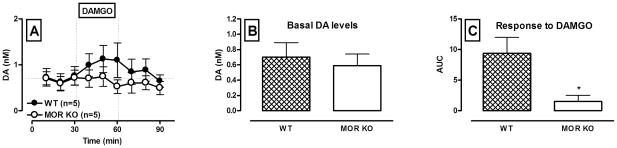
Figure 3 demonstrates basal and DAMGO-induced dopamine levels in the VTA of WT and MOR KO mice. There was no difference in basal dopamine levels between genotypes (F(1,10) = 0.22; p = 0.65; Fig. 3, A and B). Repeated measures ANOVA with one between factor (genotype) and two within factors ( drug challenge and time) showed that there was a significant main effect of DAMGO (F(1,16) = 16.13; p < 0.01) and significant genotype × drug challenge interaction (F(1,16) = 4.92; p = 0.05). The latter allowed us to probe effects of DAMGO in WT and KO animals separately. There was a significant effect of DAMGO (F(1,8) = 13.3; p = 0.02) in WT animals and no effect in KO animals (F(1,8) = 2.99; p = 0.16). KO mice did not show an elevation of dopamine levels in response to intra-VTA infusion of DAMGO (50 μM) whereas WT animals showed a significant increase in dopamine levels as compared to baseline (Fig. 3, A and C).
Figure 3.
Somatodendritic dopamine overflow during reverse-dialysis of the MOR agonist DAMGO into the VTA of WT and MOR KO mice.
A – Time course of dialysate dopamine levels before and following reverse dialysis of DAMGO (50 μM; black circles – WT, white circles – KO) into the VTA. Data are expressed as the means ± S.E.M., n - number of animals in each experimental group (5 animals per group). Other details as in Fig. 2..B – Bar graphs of basal VTA dopamine levels in WT and MOR KO mice expressed as the means ± S.E.M. C – Bar graphs of AUC values for the dopamine response to DAMGO in WT and MOR KO mice expressed as the means ± S.E.M. * denotes a significant difference in DAMGO-induced dopamine responses between WT and KO animals (p ≤ 0.05).
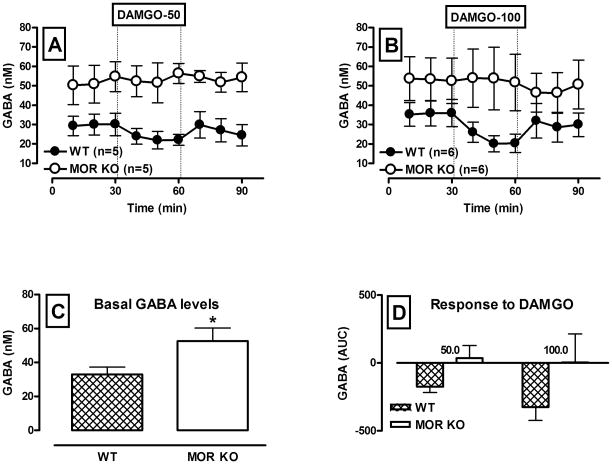
Following quantification of dopamine concentration (Fig. 3), the same microdialysis samples were analyzed for amino acid content. Basal and DAMGO-induced levels of GABA in the VTA are shown in Figure 4. Two way repeated measure ANOVA revealed a significant main effect of genotype (F(1,18) = 8.12; p = 0.010), indicating that GABA neurotransmission was notably different in WT and MOR KO mice. Thus, basal GABA levels were significantly elevated in MOR KO mice (F(1,20) = 5.05; p = 0.036 ; Fig. 4, A, B and C)). There was no change in GABA levels in response to infusion of DAMGO in KO animals (F(1,9) = 0.01; p = 0.92; Fig. 4, A,B and D). On the contrary, DAMGO decreased GABA levels in WT mice (F(1,9) = 19.94; p < 0.01; Fig. 4, A, B and D), however this effect was not concentration-dependent (Fig. 4,D), because there was no drug challenge × drug concentration (F(1,9) = 1.86; p = 0.21) or drug × drug challenge × time (F(2,18) = 0.6; p = 0.54) interaction.
Figure 4.
GABA neurotransmission during retro-dialysis of the MOR agonist DAMGO into the VTA of WT and MOR KO mice.
A and B – Time course of dialysate GABA levels before and following reverse dialysis of 50 and 100 μM DAMGO into the VTA (black circles – WT, white circles – KO). Data are expressed as the means ± S.E.M., n - number of animals in each experimental group (5 animals per group). Other details as in Fig. 2..C – Bar graphs of basal VTA GABA levels in WT and MOR KO mice expressed as the means ± S.E.M. D – Bar graphs of AUC values for the GABA response to DAMGO in WT and MOR KO mice expressed as the means ± S.E.M. * denotes a significant difference in basal GABA levels between WT and KO animals (p ≤ 0.05).
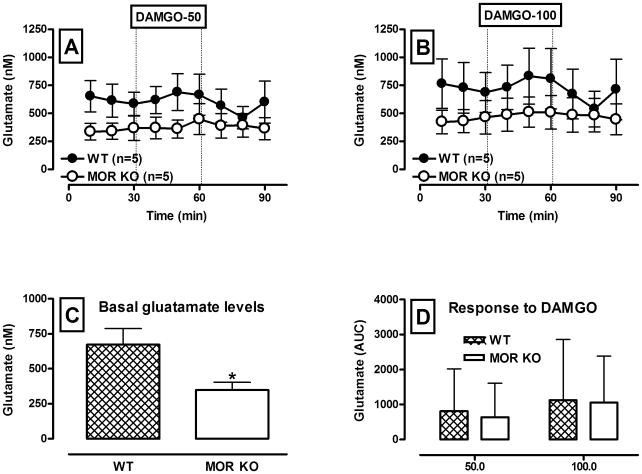
In contrast to GABA, basal glutamate concentrations were significantly lower in the VTA of MOR KO mice (F(1,20) = 4.25; p = 0.05; Fig. 5, A, B and C)). Regardless of genotype or drug concentration there were no significant changes in glutamate concentrations following intra-VTA perfusion of DAMGO via reverse microdialysis (F(1,8) = 0.49; p = 0.5 and F(1,8) = 1.97; p = 0.19 for WT and MOR KO mice, respectively; Fig. 5, A, B and D).
Figure 5.
Glutamate neurotransmission during retro-dialysis of the MOR agonist DAMGO into the VTA of WT and MOR KO mice.
A and B – Time course of dialysate glutamate levels before and following reverse dialysis of 50 and 100 μM DAMGO (black circles – WT, white circles – KO) into the VTA. Data are expressed as the means ± S.E.M., n - number of animals in each experimental group (5 animals per group). Other details as in Fig. 2..C – Bar graphs of basal VTA glutamate levels in WT and MOR KO mice expressed as the means ± S.E.M. D – Bar graphs of AUC values for the glutamate response to DAMGO in WT and MOR KO mice expressed as the means ± S.E.M. * denotes a significant difference in basal glutamate levels between WT and KO animals (p ≤ 0.05).
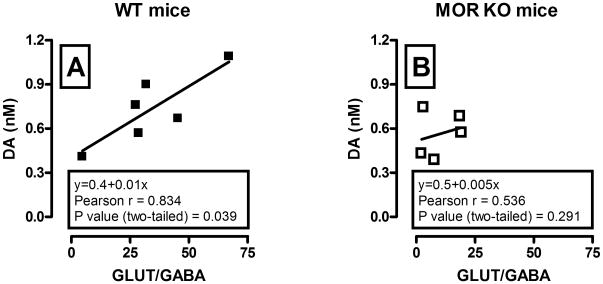
Interestingly, a correlation (Pearson’s r) analysis revealed that basal dialysate dopamine concentrations for each mouse were significantly positively correlated with basal glutamate/GABA ratio in WT (r = 0.85, P = 0.03; Fig. 6, A), but not in KO animals (r = 0.54, P = 0.29; Fig. 6, B).
Figure 6.
Correlation analysis of the relationship between basal glutamate/GABA ratio and dialysate dopamine concentrations in the VTA of WT (A) and MOR KO (B) mice. Each graph shows regression lines, regression equation, and correlation coefficient. Significant positive correlation between basal glutamate/GABA ratio and dopamine levels was observed only in WT (n = 6, r = 0.83, P < 0.05) but not in MOR KO animals (n = 5, r = 0.53, P > 0.05).
Discussion
The present studies provide the first direct neurochemical evidence that activation of MOR in the VTA of the freely moving animal produces a concentration-dependent decrease in local GABA levels and an augmentation of somatodendritic DA levels. Loss of MOR is associated with an elevation of basal GABA overflow and a reduction of glutamate levels in the VTA. No difference between genotypes in basal somatodendritic dopamine overflow was seen. However, basal dopamine levels in WT were significantly correlated with basal glutamate/GABA ratio.
There is general agreement that the firing activity of dopamine neurons is controlled by their intrinsic properties, as well as inhibitory and excitatory inputs from local neurons and from other brain areas (Johnson and North, 1992b; Kalivas, 1993; White, 1996). MOR modulation of dopamine neurons also seems to be fairly straight forward: hyperpolarization of GABA neurons due to activation of MOR located on those neurons (Bergevin et al., 2002; Garzon and Pickel, 2002) disinhibits DA neurons (Johnson and North, 1992a) resulting in an increase in their firing rate and increased DA release. However, when glutamatergic neurotransmission is taken into account this hypothesis becomes more complicated, because activation of MOR also inhibit glutamatergic inputs to dopamine and GABA neurons (Bonci and Malenka, 1999; Manzoni and Williams, 1999). Moreover, most of the data forming the basis of this hypothesis have been obtained in vitro or in anesthetized animals. The functional connectivity of these preparations differs from that in the intact or awake animal (Deleuze and Huguenard, 2006; Windels and Kiyatkin, 2006) and some of GABAergic or glutamatergic influences may not be detected. To our knowledge, no study has been performed to date to investigate MOR-induced changes in GABA and glutamate neurotransmission in the VTA of the free moving animals.
The results of the present study, although not unexpected, provide the first definitive demonstration that the selective activation of MOR in the VTA decreases GABA release in unanesthetized animals. These responses were not observed in MOR KO animals, indicating that these effects of DAMGO were MOR-mediated. More over, changes in GABA outflow were associated with concentration-dependent increases in somatodendritic dopamine levels, confirming the results of electrophysiological and morphological studies (Bergevin et al., 2002; Garzon and Pickel, 2002).
Electrophysiological experiments have shown that MOR agonists depress glutamatergic inputs to both dopamine and GABA neurons in the VTA (Bonci and Malenka, 1999; Manzoni and Williams, 1999). However, in the present study reverse dialysis of DAMGO did not change VTA glutamate levels. It is worth to mention that microdialysis may fail to sample synaptic pools of glutamate due to rapid clearance of this neurotransmitter by its uptake carrier and relatively high levels of non-neuronal glutamate in extracellular space. Both these factors can obscure drug-induced alterations in glutamate overflow. Often increases in dialysate glutamate levels can be seen only when its uptake is blocked. Thus, in a recent paper Schepers et al. (Schepers et al., 2008) showed that infusion of DAMGO increased glutamate concentrations in the rostral ventromedial medulla only in the presence of the selective glutamate transport inhibitor tPDC. In these experiments reversed dialysis of tPDC increased basal glutamate levels and allowed the detection of depolarization-evoked glutamate overflow. However, blockade of glutamate uptake with tPDC would not unravel acute inhibition of glutamate release by MOR agonist expected from electrophysiological studies, because it will be masked by high glutamate levels in extracellular space.
Constitutive deletion of MOR resulted in elevated basal levels of GABA and reduced glutamate levels in the VTA. Such changes in basal neurotransmitter levels indicate increased inhibitory tone in the absence of MOR. This result corroborates the existence of a tonically active MOR system in the VTA (Spanagel et al., 1992). Furthermore it is in accord with electrophysiological data showing that the frequency of spontaneous inhibitory post-synaptic currents (sIPSCs) onto dopaminergic neurons is higher in MOR KO mice as compared to WT animals (Mathon et al., 2005a). However, the firing activity of dopamine and GABA neurons was found unaltered in this study. This may be due to lack of some functional connectivity and washout of critical intracellular components in brain slice preparations. Thus, in vivo experiments demonstrated that MOR KO mice have lower firing activity of dopamine neurons (Mathon et al., 2005b). Decreased impulse activity of dopamine neurons substantiates reported previously indirect evidence for decreased dopamine release in the NAc of the MOR KO mice (Chefer et al., 2003).
The results of the present study show that in mice lacking MOR the basal level of dopamine in the VTA is not significantly different from WT animals. This finding may, at first glance, appear inconsistent with the low firing activity of dopamine neurons (Mathon et al., 2005b) and decreased dopamine release in the NAc (Chefer et al., 2003) observed in MOR KO mice in vivo. However, in the present study somatodendritic rather than terminal dopamine levels were assessed. Somatodendritic and terminal release of dopamine in the mesocorticolimbic system are largely dependent on impulse activity in the VTA (Kalivas and Duffy, 1991, Adell and Artigas, 2004). However, the relationship between somatodendritic dopamine release and firing of dopaminergic neurons is not simple, and dendritic release may occur independently of neuronal firing (see Bustos et al., 2004 for review). Basal levels of dopamine also depend on population activity (i.e. the number of spontaneously active cells; see Floresco et al., 2003). Interestingly, studies in terminal regions have shown that stimulation of dopamine fibers/cells activates them synchronously, thereby, increasing population activity, as well as burst-firing. Whether this is also the case at the level of the dopamine cell body is unknown and warrants a study.
Overall, the relationship between impulse flow and somatodendritic DA release has not yet been properly established. Importantly, however, the present findings suggest that during local application of the MOR agonist DAMGO, the resulting disinhibition of DA neurons is reflected in augmentation of somatodendritic DA levels. However, in the case of constitutive deletion of MOR long-term changes in neuronal activity are not mirrored by changes in somatodendritic dopamine, perhaps due to developmental compensations in KO animals.
An advantage of the present study is the fact that DA, glutamate and GABA were measured simultaneously in the same animals; thereby allowing assessment of the interrelationship of these neurotransmitters. Importantly, there was a significant positive correlation between basal somatodendritic DA levels and glutamate/GABA ratio in WT, but not in KO animals. This indicates that DA neurotransmission in the VTA is normally maintained by an intricate balance between glutamatergic and GABAergic neurotransmission. In the absence of MOR, this balance is disrupted.
In accordance with the involvement of MOR in drug reinforcement, MOR KO mice demonstrate reduced sensitivity to the reinforcing properties of various drugs of abuse including morphine, heroin, alcohol, Δ9-tetrahydrocannabinol, nicotine and cocaine (Kieffer and Gaveriaux-Ruff, 2002). This reduction may be attributed, at least in part, to the dysregulation of the intricate balance between glutamatergic and GABAergic neurotransmission in the VTA observed in the present study, the resultant augmentation in the frequency of sIPSCs (Mathon et al., 2005a), and corresponding decreases in firing activity of dopamine neurons (Mathon et al., 2005b) and dopamine release in the NAc (Chefer et al., 2003). Therefore the current findings add new evidence that VTA MOR may contribute to addiction vulnerability, by modulating GABA and glutamatergic inputs to dopamine neurons (Mathon et al., 2005a).
In summary, the present studies demonstrate that activation of MOR in the VTA decreases GABA and increases dopamine overflow in the VTA of unanesthetized animals. Furthermore, there is a positive correlation between basal dopamine levels and the glutamate/GABA ratio in the VTA of WT animals. In the absence of MOR, inhibitory tone in the VTA is significantly enhanced and excitatory tone is lessened. Importantly, there is no correlation between dopaminergic and glutamate/GABA neurotransmission in the VTA in animals lacking MOR, which suggests a lack of balance between excitatory and inhibitory inputs to dopaminergic neurons under this condition. The modulatory effects of VTA MOR on GABA and glutamate neurotransmission provide potential new insights as to the mechanisms by which targeting MOR system can attenuate the rewarding effects of various drugs of abuse, regardless of their pharmacological class.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse. The experiments comply with the current laws of the US, and the authors have no financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- KO
knockout mice
- MOR
mu opioid receptor
- NAc
nucleus accumbens
- NO-711
-(((Diphenylmethylene)imino)oxy)ethyl)-1,2,5,6-tetrahydro-3-pyridine-carboxylic acid
- tPDC
L-trans-pyrrolidine-2,4-dicarboxylic acid
- VTA
ventral tegmental area
- WT
wildtype mice
References
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev. 2004;28(4):415–431. doi: 10.1016/j.neubiorev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bergevin A, Girardot D, Bourque MJ, Trudeau LE. Presynaptic mu-opioid receptors regulate a late step of the secretory process in rat ventral tegmental area GABAergic neurons. Neuropharmacology. 2002;42:1065–1078. doi: 10.1016/s0028-3908(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Involvement of the ventral tegmental dopamine system in opioid and psychomotor stimulant reinforcement. NIDA Res Monogr. 1986;67:190–196. [PubMed] [Google Scholar]
- Bustos G, Abarca J, Campusano J, Bustos V, Noriega V, Aliaga E. Functional interactions between somatodendritic dopamine release, glutamate receptors and brain-derived neurotrophic factor expression in mesencephalic structures of the brain. Brain Res Brain Res Rev. 2004;47(1–3):126–144. doi: 10.1016/j.brainresrev.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Kieffer BL, Shippenberg TS. Basal and morphine-evoked dopaminergic neurotransmission in the nucleus accumbens of MOR- and DOR-knockout mice. Eur J Neurosci. 2003;18:1915–1922. doi: 10.1046/j.1460-9568.2003.02912.x. [DOI] [PubMed] [Google Scholar]
- David V, Cazala P. A comparative study of self-administration of morphine into the amygdala and the ventral tegmental area in mice. Behav Brain Res. 1994;65:205–211. doi: 10.1016/0166-4328(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Deleuze C, Huguenard JR. Distinct electrical and chemical connectivity maps in the thalamic reticular nucleus: potential roles in synchronization and sensation. J Neurosci. 2006;26:8633–8645. doi: 10.1523/JNEUROSCI.2333-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DP, Leone P, Pocock D, Wise RA. Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther. 1993;266:1236–1246. [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci. 1994;14:1978–1984. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilts RP, Kalivas PW. Autoradiographic localization of mu-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res. 1989;488:311–327. doi: 10.1016/0006-8993(89)90723-3. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003 Sep;6(9):968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Garzon M, Pickel VM. Plasmalemmal mu-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse. 2001;41:311–328. doi: 10.1002/syn.1088. [DOI] [PubMed] [Google Scholar]
- Garzon M, Pickel VM. Ultrastructural localization of enkephalin and mu-opioid receptors in the rat ventral tegmental area. Neuroscience. 2002;114:461–474. doi: 10.1016/s0306-4522(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992a;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurons in the rat ventral tegmental area and their synaptic inputs. J Physiol (Lond ) 1992b;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. A comparison of axonal and somatodendritic dopamine release using in vivo dialysis. J Neurochem. 1991;56(3):961–967. doi: 10.1111/j.1471-4159.1991.tb02015.x. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Liachenko S, Tang P, Somogyi GT, Xu Y. Concentration-dependent isoflurane effects on depolarization-evoked glutamate and GABA outflows from mouse brain slices. Br J Pharmacol. 1999;127(1):131–138. doi: 10.1038/sj.bjp.0702543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathon DS, Lesscher HM, Gerrits MA, Kamal A, Pintar JE, Schuller AG, Spruijt BM, Burbach JP, Smidt MP, van Ree JM, Ramakers GM. Increased gabaergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice. Neuroscience. 2005a;130:359–367. doi: 10.1016/j.neuroscience.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Mathon DS, Ramakers GM, Pintar JE, Marinelli M. Decreased firing frequency of midbrain dopamine neurons in mice lacking mu opioid receptors. Eur J Neurosci. 2005b;21:2883–2886. doi: 10.1111/j.1460-9568.2005.04123.x. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Phillips AG, Le Piane FG. Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol Biochem Behav. 1980;12:965–968. doi: 10.1016/0091-3057(80)90460-8. [DOI] [PubMed] [Google Scholar]
- Rada P, Mark GP, Pothos E, Hoebel BG. Systemic morphine simultaneously decreases extracellular acetylcholine and increases dopamine in the nucleus accumbens of freely moving rats. Neuropharmacology. 1991;30:1133–1136. doi: 10.1016/0028-3908(91)90145-2. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Zapata A, Chefer V, Shippenberg TS. The effects of local perfusion of DAMGO on extracellular GABA and glutamate concentrations in the rostral ventromedial medulla. J Neurochem. 2008;104:806–817. doi: 10.1111/j.1471-4159.2007.05017.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7:191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Kamiyama Y, Hüttemeier PC, Benveniste H. Extracellular glutamate and dopamine measured by microdialysis in the rat striatum during blockade of synaptic transmission in anesthetized and awake rats. Brain Res. 1997;759(2):221–227. doi: 10.1016/s0006-8993(97)00258-8. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Identification of the opioid receptor types mediating beta-endorphin- induced alterations in dopamine release in the nucleus accumbens. Eur J Pharmacol. 1990a;190:177–184. doi: 10.1016/0014-2999(90)94124-g. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990b;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Garzon M, Colago EE, Pickel VM. Mu-opioid receptors in the ventral tegmental area are targeted to presynaptically and directly modulate mesocortical projection neurons. Synapse. 2001;41:221–229. doi: 10.1002/syn.1079. [DOI] [PubMed] [Google Scholar]
- White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Annu Rev Neurosci. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]
- Windels F, Kiyatkin EA. General anesthesia as a factor affecting impulse activity and neuronal responses to putative neurotransmitters. Brain Res. 2006;1086:104–116. doi: 10.1016/j.brainres.2006.02.064. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]