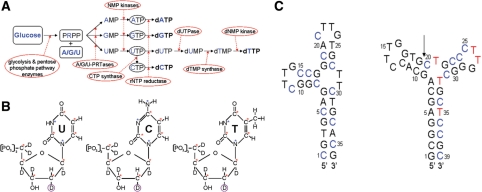
Figure 2.
(A) In vitro (deoxy)ribonucleotide triphosphate synthesis. Labeled starting materials are shown in bold and blue. Labeled rNTPs, here as intermediate products, are circled. Labeled end-product dNTPs are shown in bold. The enzymes catalyzing different reactions are circled. See text for detailed description of the reactions. (B) Overview of the in vitro synthesized labeled dNTPs. From left to right: 13C9/15N2/2H(1′,2″,3′,4′,5′,5″)-dUTP, 13C9/15N3/2H(1′,2″,3′,4′,5′,5″)-dCTP and 13C9/15N2/2H(1′,2″,3′,4′,5′,5″)-dTTP. The red asterisks at carbon atoms indicate 13C labels; the blue asterisks at nitrogen atoms indicate 15N labels. The circled deuteron indicates the introduced stereo-selective deuteration in the sugar moiety during rNTP reduction. (C) Predicted most stable secondary structures of the three-way junction ssDNA (left) and the triple-repeat three-way junction ssDNA (junction 6 wt, right). The arrow indicates the chosen segmentation site in junction 6 wt. Blue cytidine residues are 13C9/15N3/2H(1′,2″,3′,4′,5′,5″)-labeled (see also B) and red thymidine residues are 13C9/15N2/2H(1′,2″,3′,4′,5′,5″). (see B).