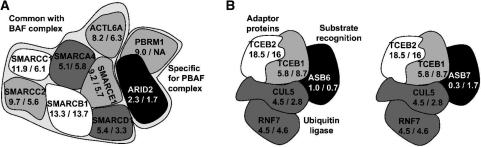
Figure 5.
RNA half-lives for the PBAF (A) and ubiquitin ligase (B) complexes. Half-lives (in hours) for human/mouse are indicated and mapped to grey scales ranging from black (short RNA half-lives) to white (long RNA half-lives). For PBRM1, no transcript half-life could be obtained for murine fibroblasts. For SMARCD1, its RNA half-life in murine fibroblasts was taken from 30 min labeling experiments (14). (A) The PBAF complex consists of several proteins in common with the BAF complex and two specific proteins (ARID2 and PBRM1) (45). ARID2, the only subunit with short transcript half-life in this complex, has been found to be essential for complex stability, potentially by recruiting PBRM1 (45). As most physical interactions in the complex are not characterized, the arrangement of the proteins in this figure does not necessarily represent the true complex structure. (B) Substrate-specificity of the ubiquitin ligase containing CUL5 and RNF7 is determined by binding to different ASB proteins (46,47). TCEB1 and TCEB2 are adaptor proteins which form a heterodimeric complex (Elongin BC) and additionally link the ligase subunits (CUL5 and RNF7) and the ASB protein. Short transcript half-life of the ASB6 and ASB7 subunits allows efficient regulation of complex activity with regard to specific substrates.