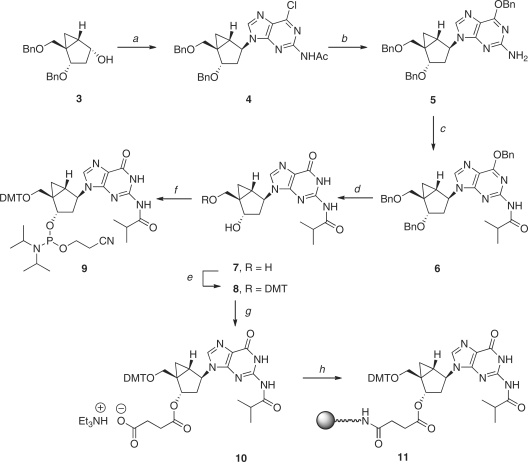
Scheme 1.
Reagents and conditions: (a) PPh3, DIAD, 2-acetamide-6-chrolopurine, THF, 0°C, 73%; (b) BnONa, BnOH, rt, 89%; (c) isobutyryl chloride, pyridine, rt, 84%; (d) BCl3, CH2Cl2, –78°C, 69%; (e) DMTr-Cl, pyridine, rt, 83%; (f) Diisopropylethylamine, 2-cyanoethyl diisopropylchlorophosphoramidite, CH2Cl2 (g) Succinic anhydride, Et3N, DMAP, CH2Cl2, rt, 81%; (h) i. LCAA-CPG, DCC, CH2Cl2; ii. pyridine:Ac2O (9:1, v/v), DMAP.