Abstract
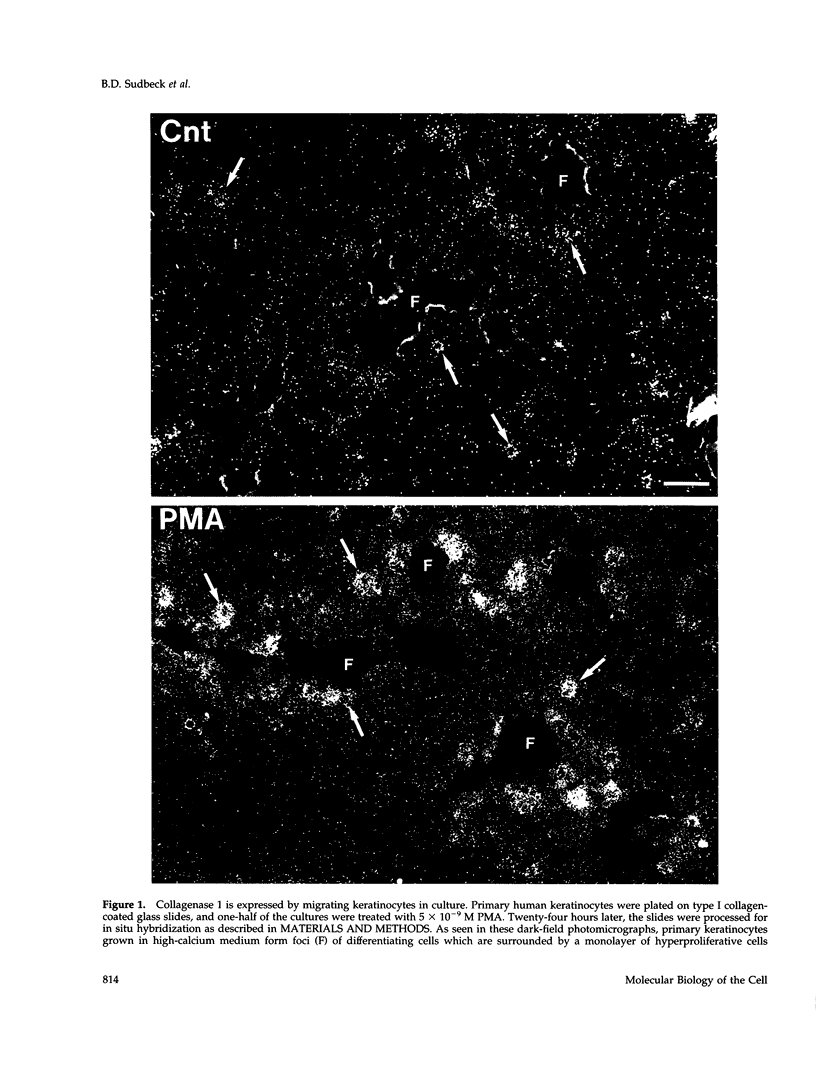
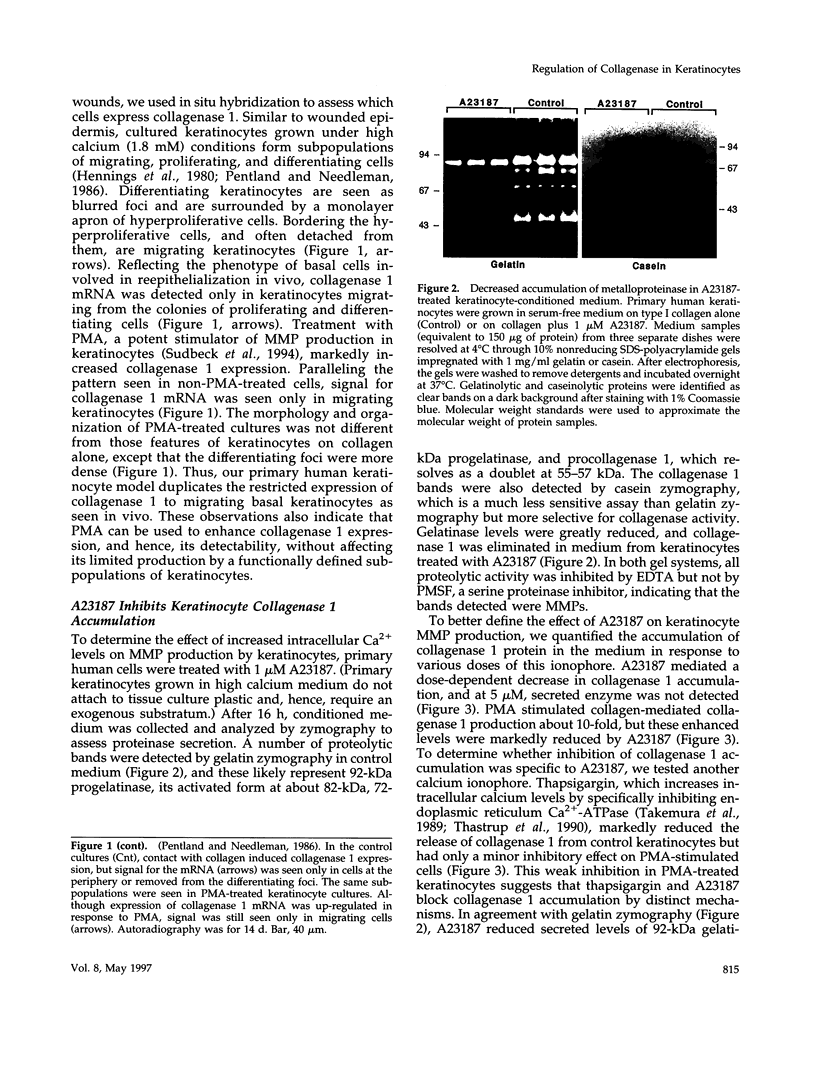
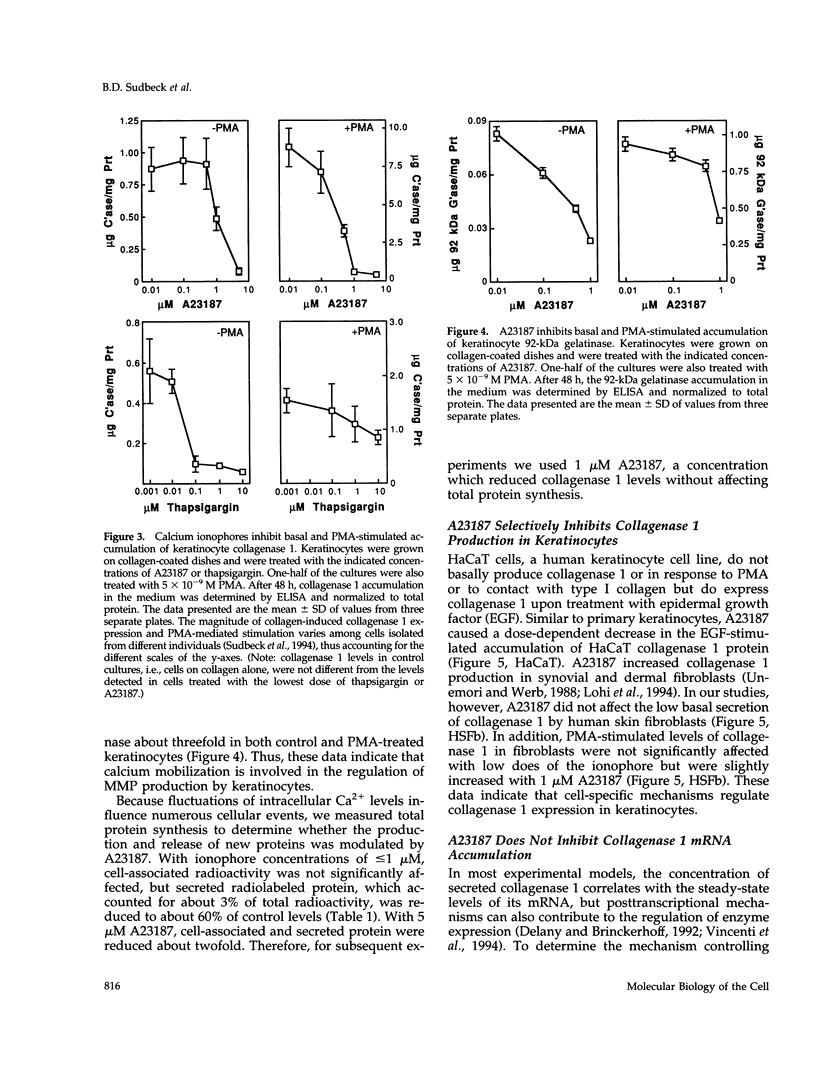
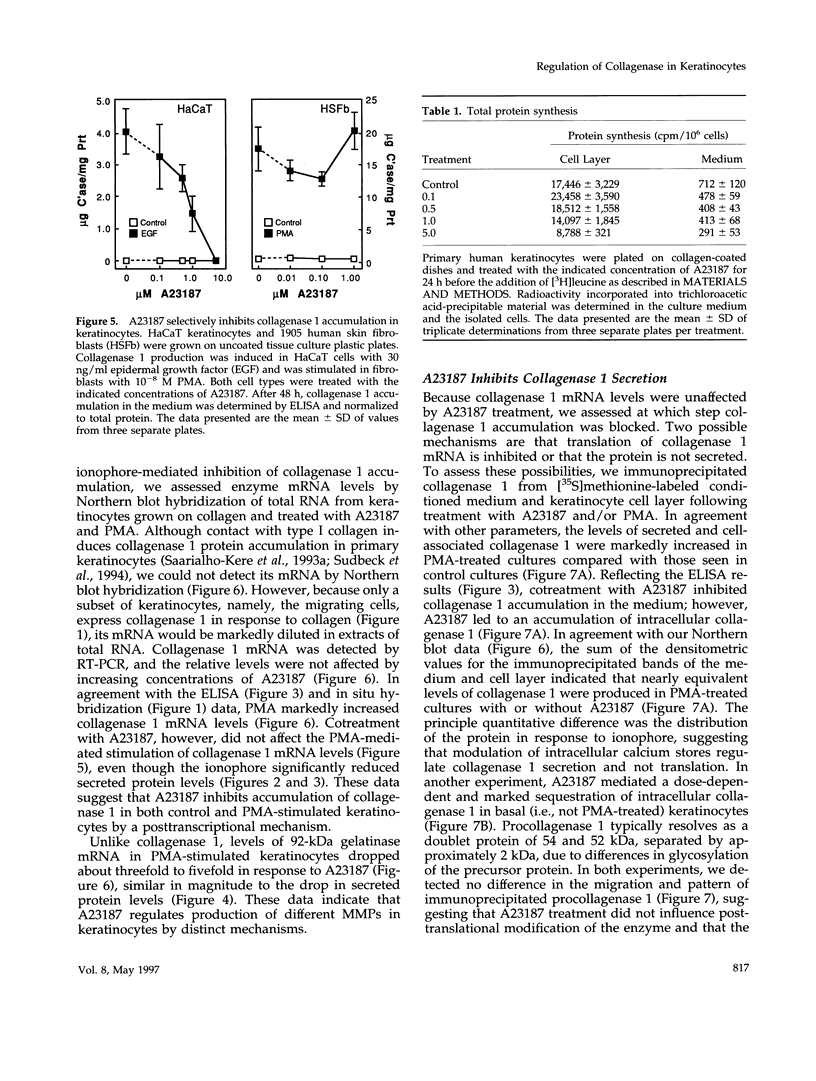
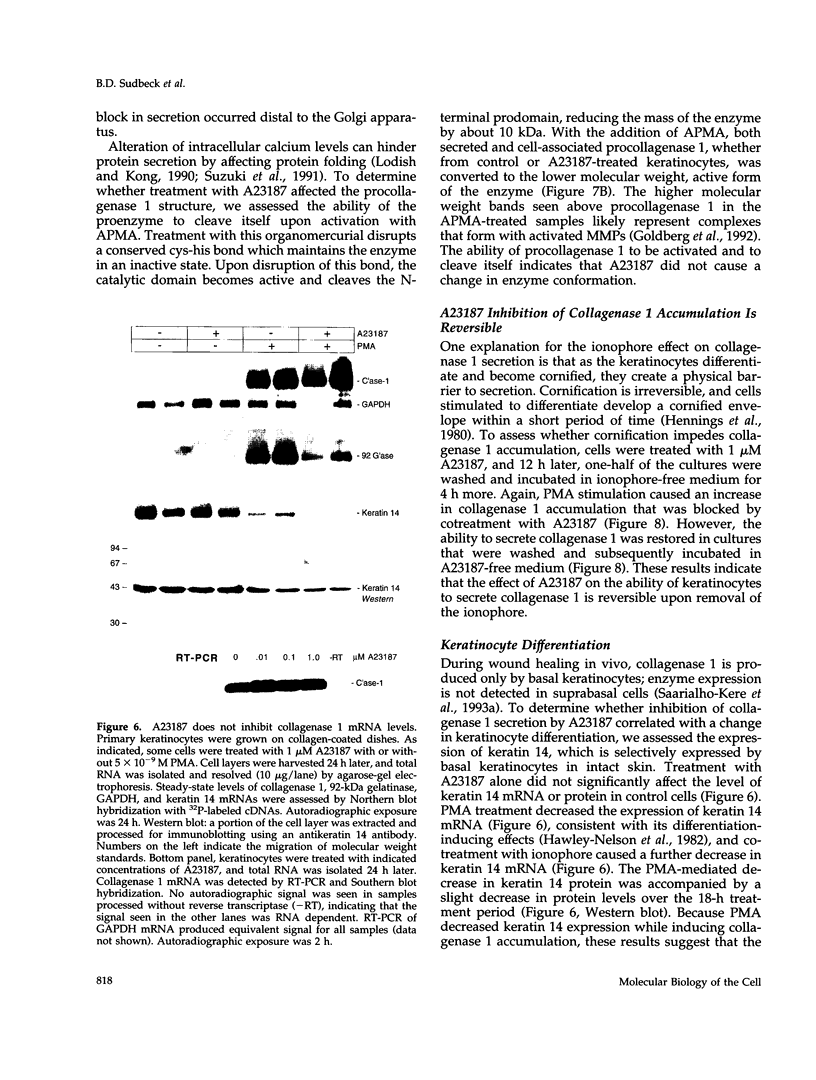
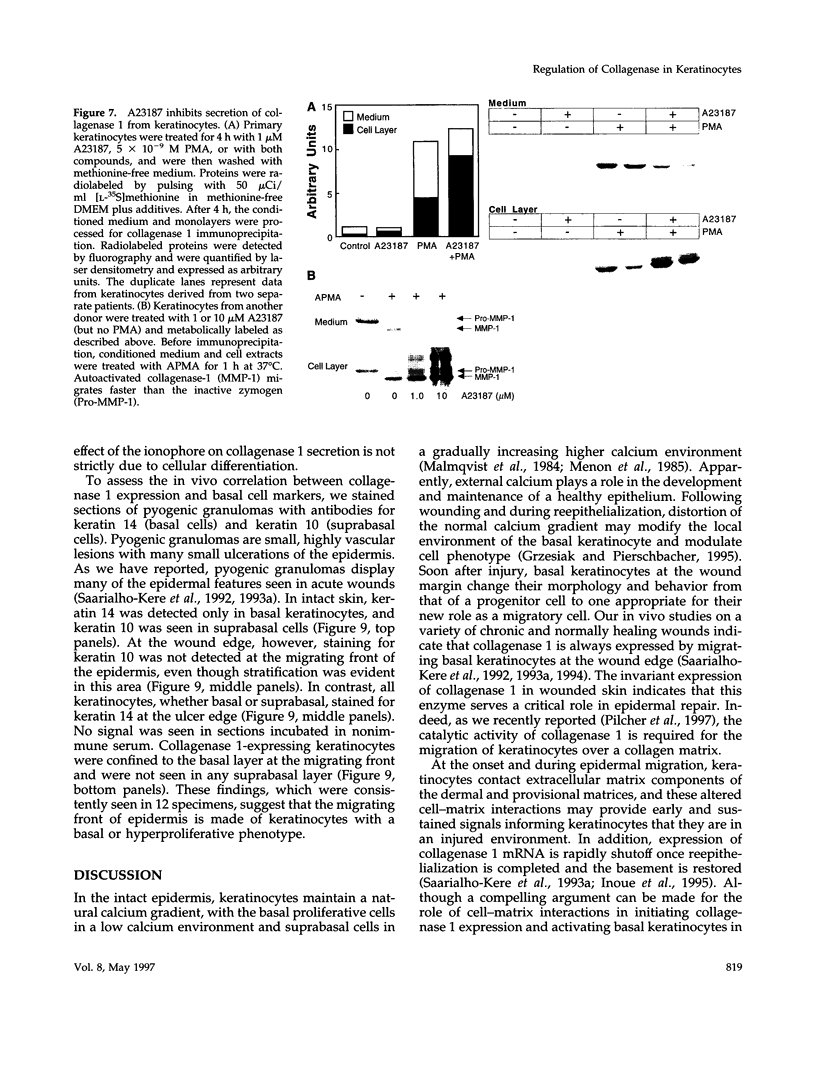
Calcium concentration influences keratinocyte differentiation, and, following injury, keratinocytes move through an environment of changing calcium levels. Because these migrating cells in wounds invariably express collagenase 1, we assessed if modulation of calcium levels regulates collagenase 1 production by primary human keratinocytes. Accurately reflecting the confined spatial pattern of enzyme production seen in vivo, collagenase 1 mRNA was expressed only by keratinocytes migrating from foci of differentiated cells. Treatment with calcium ionophores A23187 or thapsigargin markedly inhibited the basal and phorbol 12-myristate 13-acetate-(PMA) stimulated accumulation of keratinocyte collagenase 1 in the medium but did not affect collagenase 1 production by control or PMA-treated fibroblasts. A23187-mediated inhibition of collagenase 1 protein was not associated with a decrease in mRNA levels but rather was controlled by a selective and reversible block of enzyme secretion. This block in secretion was likely not due to altered protein folding as the proenzyme within A23187-treated cells remained capable of autolytic activation upon treatment with p-aminophenylmercuric acetate. In contrast, 92-kDa gelatinase mRNA and secreted protein levels were coordinately reduced by A23187. Keratin 14 expression, a basal keratinocyte marker, was reduced with PMA treatment, but A23187 did not affect keratin 14 expression. In human wounds, both basal and suprabasal keratinocytes at the migrating front of epidermis stained for keratin 14, but only the basal cells expressed collagenase 1. These data suggest that collagenase 1 production is not necessarily linked with expression of basal cell markers and that modulation of intracellular calcium levels can block secretion of collagenase 1 by keratinocytes which have moved away from the stratum basalis and from their natural substrate.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkedal-Hansen H., Moore W. G., Bodden M. K., Windsor L. J., Birkedal-Hansen B., DeCarlo A., Engler J. A. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busiek D. F., Baragi V., Nehring L. C., Parks W. C., Welgus H. G. Matrilysin expression by human mononuclear phagocytes and its regulation by cytokines and hormones. J Immunol. 1995 Jun 15;154(12):6484–6491. [PubMed] [Google Scholar]
- Carroll J. M., Romero M. R., Watt F. M. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995 Dec 15;83(6):957–968. doi: 10.1016/0092-8674(95)90211-2. [DOI] [PubMed] [Google Scholar]
- D'Armiento J., DiColandrea T., Dalal S. S., Okada Y., Huang M. T., Conney A. H., Chada K. Collagenase expression in transgenic mouse skin causes hyperkeratosis and acanthosis and increases susceptibility to tumorigenesis. Mol Cell Biol. 1995 Oct;15(10):5732–5739. doi: 10.1128/mcb.15.10.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany A. M., Brinckerhoff C. E. Post-transcriptional regulation of collagenase and stromelysin gene expression by epidermal growth factor and dexamethasone in cultured human fibroblasts. J Cell Biochem. 1992 Dec;50(4):400–410. doi: 10.1002/jcb.240500409. [DOI] [PubMed] [Google Scholar]
- Desrochers P. E., Jeffrey J. J., Weiss S. J. Interstitial collagenase (matrix metalloproteinase-1) expresses serpinase activity. J Clin Invest. 1991 Jun;87(6):2258–2265. doi: 10.1172/JCI115262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini M. E., Parks W. C., Rinehart W. B., Girard M. T., Matsubara M., Cook J. R., West-Mays J. A., Sadow P. M., Burgeson R. E., Jeffrey J. J. Role of matrix metalloproteinases in failure to re-epithelialize after corneal injury. Am J Pathol. 1996 Oct;149(4):1287–1302. [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990 Dec;111(6 Pt 2):2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Strongin A., Collier I. E., Genrich L. T., Marmer B. L. Interaction of 92-kDa type IV collagenase with the tissue inhibitor of metalloproteinases prevents dimerization, complex formation with interstitial collagenase, and activation of the proenzyme with stromelysin. J Biol Chem. 1992 Mar 5;267(7):4583–4591. [PubMed] [Google Scholar]
- Goldberg G. I., Wilhelm S. M., Kronberger A., Bauer E. A., Grant G. A., Eisen A. Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986 May 15;261(14):6600–6605. [PubMed] [Google Scholar]
- Grzesiak J. J., Pierschbacher M. D. Shifts in the concentrations of magnesium and calcium in early porcine and rat wound fluids activate the cell migratory response. J Clin Invest. 1995 Jan;95(1):227–233. doi: 10.1172/JCI117644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpert I., Sires U. I., Roby J. D., Potter-Perigo S., Wight T. N., Shapiro S. D., Welgus H. G., Wickline S. A., Parks W. C. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley-Nelson P., Stanley J. R., Schmidt J., Gullino M., Yuspa S. H. The tumor promoter, 12-O-tetradecanoylphorbol-13-acetate accelerates keratinocyte differentiation and stimulates growth of an unidentified cell type in cultured human epidermis. Exp Cell Res. 1982 Jan;137(1):155–167. doi: 10.1016/0014-4827(82)90017-9. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Herron G. S., Banda M. J., Clark E. J., Gavrilovic J., Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986 Feb 25;261(6):2814–2818. [PubMed] [Google Scholar]
- Hertle M. D., Kubler M. D., Leigh I. M., Watt F. M. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992 Jun;89(6):1892–1901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin N. A., Watt F. M. Transcriptional and post-translational regulation of beta 1 integrin expression during keratinocyte terminal differentiation. J Biol Chem. 1992 Jul 25;267(21):14852–14858. [PubMed] [Google Scholar]
- Inoue M., Kratz G., Haegerstrand A., Ståhle-Bäckdahl M. Collagenase expression is rapidly induced in wound-edge keratinocytes after acute injury in human skin, persists during healing, and stops at re-epithelialization. J Invest Dermatol. 1995 Apr;104(4):479–483. doi: 10.1111/1523-1747.ep12605917. [DOI] [PubMed] [Google Scholar]
- Juhasz I., Murphy G. F., Yan H. C., Herlyn M., Albelda S. M. Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol. 1993 Nov;143(5):1458–1469. [PMC free article] [PubMed] [Google Scholar]
- Kallioinen M., Koivukangas V., Järvinen M., Oikarinen A. Expression of cytokeratins in regenerating human epidermis. Br J Dermatol. 1995 Dec;133(6):830–835. doi: 10.1111/j.1365-2133.1995.tb06912.x. [DOI] [PubMed] [Google Scholar]
- Kubler M. D., Watt F. M. Changes in the distribution of actin-associated proteins during epidermal wound healing. J Invest Dermatol. 1993 Jun;100(6):785–789. doi: 10.1111/1523-1747.ep12476492. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990 Jul 5;265(19):10893–10899. [PubMed] [Google Scholar]
- Lohi J., Keski-Oja J. Calcium ionophores decrease pericellular gelatinolytic activity via inhibition of 92-kDa gelatinase expression and decrease of 72-kDa gelatinase activation. J Biol Chem. 1995 Jul 21;270(29):17602–17609. doi: 10.1074/jbc.270.29.17602. [DOI] [PubMed] [Google Scholar]
- Lohi J., Kähäri V. M., Keski-Oja J. Cyclosporin A enhances cytokine and phorbol ester-induced fibroblast collagenase expression. J Invest Dermatol. 1994 Jun;102(6):938–944. doi: 10.1111/1523-1747.ep12384105. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M., McDonnell S., Miller D. B., Navre M., Seftor E. A., Hendrix M. J. The role of the matrix metalloproteinase stromelysin in the progression of squamous cell carcinomas. Am J Med Sci. 1991 Sep;302(3):157–162. doi: 10.1097/00000441-199109000-00008. [DOI] [PubMed] [Google Scholar]
- Mauviel A., Chung K. Y., Agarwal A., Tamai K., Uitto J. Cell-specific induction of distinct oncogenes of the Jun family is responsible for differential regulation of collagenase gene expression by transforming growth factor-beta in fibroblasts and keratinocytes. J Biol Chem. 1996 May 3;271(18):10917–10923. doi: 10.1074/jbc.271.18.10917. [DOI] [PubMed] [Google Scholar]
- Menon G. K., Grayson S., Elias P. M. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985 Jun;84(6):508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- Parks W. C., Secrist H., Wu L. C., Mecham R. P. Developmental regulation of tropoelastin isoforms. J Biol Chem. 1988 Mar 25;263(9):4416–4423. [PubMed] [Google Scholar]
- Pei D., Weiss S. J. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J Biol Chem. 1996 Apr 12;271(15):9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- Pentland A. P., Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986 Jan;77(1):246–251. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser I. W., Stenmark K. R., Suthar M., Crouch E. C., Mecham R. P., Parks W. C. Regional heterogeneity of elastin and collagen gene expression in intralobar arteries in response to hypoxic pulmonary hypertension as demonstrated by in situ hybridization. Am J Pathol. 1989 Dec;135(6):1073–1088. [PMC free article] [PubMed] [Google Scholar]
- Rømer J., Lund L. R., Eriksen J., Ralfkiaer E., Zeheb R., Gelehrter T. D., Danø K., Kristensen P. Differential expression of urokinase-type plasminogen activator and its type-1 inhibitor during healing of mouse skin wounds. J Invest Dermatol. 1991 Nov;97(5):803–811. doi: 10.1111/1523-1747.ep12486833. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Chang E. S., Welgus H. G., Parks W. C. Distinct localization of collagenase and tissue inhibitor of metalloproteinases expression in wound healing associated with ulcerative pyogenic granuloma. J Clin Invest. 1992 Nov;90(5):1952–1957. doi: 10.1172/JCI116073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Crouch E. C., Parks W. C. Matrix metalloproteinase matrilysin is constitutively expressed in adult human exocrine epithelium. J Invest Dermatol. 1995 Aug;105(2):190–196. doi: 10.1111/1523-1747.ep12317104. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Kovacs S. O., Pentland A. P., Olerud J. E., Welgus H. G., Parks W. C. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993 Dec;92(6):2858–2866. doi: 10.1172/JCI116906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Pentland A. P., Birkedal-Hansen H., Parks W. C., Welgus H. G. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J Clin Invest. 1994 Jul;94(1):79–88. doi: 10.1172/JCI117351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Welgus H. G., Parks W. C. Distinct mechanisms regulate interstitial collagenase and 92-kDa gelatinase expression in human monocytic-like cells exposed to bacterial endotoxin. J Biol Chem. 1993 Aug 15;268(23):17354–17361. [PubMed] [Google Scholar]
- Sank A., Chi M., Shima T., Reich R., Martin G. R. Increased calcium levels alter cellular and molecular events in wound healing. Surgery. 1989 Dec;106(6):1141–1148. [PubMed] [Google Scholar]
- Sharpe G. R., Fisher C., Gillespie J. I., Greenwell J. R. Growth and differentiation stimuli induce different and distinct increases in intracellular free calcium in human keratinocytes. Arch Dermatol Res. 1993;284(8):445–450. doi: 10.1007/BF00373354. [DOI] [PubMed] [Google Scholar]
- Stricklin G. P., Li L., Jancic V., Wenczak B. A., Nanney L. B. Localization of mRNAs representing collagenase and TIMP in sections of healing human burn wounds. Am J Pathol. 1993 Dec;143(6):1657–1666. [PMC free article] [PubMed] [Google Scholar]
- Sudbeck B. D., Parks W. C., Welgus H. G., Pentland A. P. Collagen-stimulated induction of keratinocyte collagenase is mediated via tyrosine kinase and protein kinase C activities. J Biol Chem. 1994 Nov 25;269(47):30022–30029. [PubMed] [Google Scholar]
- Suzuki C. K., Bonifacino J. S., Lin A. Y., Davis M. M., Klausner R. D. Regulating the retention of T-cell receptor alpha chain variants within the endoplasmic reticulum: Ca(2+)-dependent association with BiP. J Cell Biol. 1991 Jul;114(2):189–205. doi: 10.1083/jcb.114.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swee M. H., Parks W. C., Pierce R. A. Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. J Biol Chem. 1995 Jun 23;270(25):14899–14906. doi: 10.1074/jbc.270.25.14899. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Folmer J., Coulombe P. A. Increased expression of keratin 16 causes anomalies in cytoarchitecture and keratinization in transgenic mouse skin. J Cell Biol. 1994 Oct;127(2):505–520. doi: 10.1083/jcb.127.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unemori E. N., Werb Z. Collagenase expression and endogenous activation in rabbit synovial fibroblasts stimulated by the calcium ionophore A23187. J Biol Chem. 1988 Nov 5;263(31):16252–16259. [PubMed] [Google Scholar]
- Van Wart H. E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti M. P., Coon C. I., Lee O., Brinckerhoff C. E. Regulation of collagenase gene expression by IL-1 beta requires transcriptional and post-transcriptional mechanisms. Nucleic Acids Res. 1994 Nov 11;22(22):4818–4827. doi: 10.1093/nar/22.22.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. L., Heppner K. J., Rudolph L. A., Matrisian L. M. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol Biol Cell. 1995 Jul;6(7):851–869. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Kilkenny A. E., Steinert P. M., Roop D. R. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989 Sep;109(3):1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]