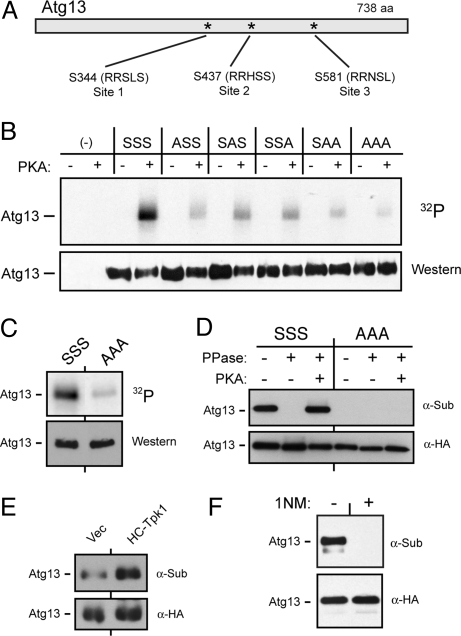
Fig. 2.
The Atg13 protein was a substrate for PKA. (A) The three conserved sites of PKA phosphorylation in Atg13 are shown. (B and C) The in vitro phosphorylation of Atg13 was dependent upon the presence of the above three PKA sites. The indicated Atg13 variants were precipitated from yeast cells and incubated with [γ-32P] ATP and either bovine PKA (bPKA) (in B) or the S. cerevisiae Tpk1 (C). S, serine; A, alanine. (D) The three PKA sites were required for the in vivo phosphorylation of Atg13. The indicated Atg13 proteins were immunoprecipitated from yeast cell extracts with an α-HA antibody and treated with λ phosphatase and then incubated with bPKA and 3 mM ATP, as indicated. The level of PKA phosphorylation was assessed by Western blotting with an α-substrate antibody that recognizes phosphorylated PKA sites. (E) The in vivo level of PKA phosphorylation on Atg13 was elevated in a strain over-expressing Tpk1. (F) Recognition by the α-substrate antibody was lost following inactivation of PKA. The tpk1-as strain was incubated with 10 μM 1NM-PP1 for 4 h and the PKA phosphorylation level of Atg13 was assessed by Western blotting with the α-substrate antibody.