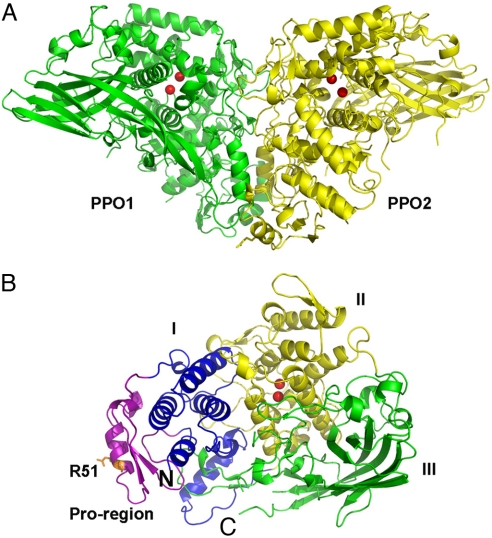
Fig. 1.
Overall structure of M. sexta PPO. (A) The heterodimeric PPO is formed in a back-to-back mode. PPO1 and PPO2 are shown in green and yellow, respectively. (B) Domains of PPO2 are colored as follows: pro-region, purple; domain I, blue; domain II, yellow; domain III, green. The di-copper atoms are located in domain II and are shown as red spheres. The proteolytic site R51 residue is shown in the stick. The amino-terminus and carboxyl-terminus of PPO2 are indicated as N and C, respectively.