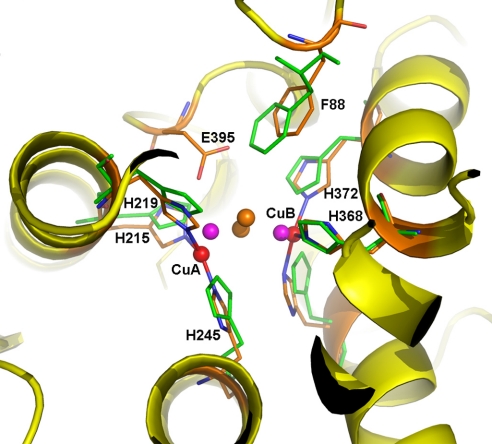
Fig. 2.
Di-copper center in M. sexta PPO2. The active site of PPO2 can be superimposed well with that of oxygenated Limulus polyphemus hemocyanin (Lp-HC, PDB ID code 1OXY). The secondary structures of PPO2 are shown in the ribbon and colored in yellow. The 6 copper-coordinating His ligands are shown as sticks, with those from Lp-HC colored green. The di-copper atoms are shown as spheres: PPO2, red; Lp-HC, purple. The peroxide ion in Lp-HC is shown as brown spheres. Notice the unique E395 in PPO2, which is located near the substrate place holder F88. E395 could be a base for phenol deprotonation, which is key to the ortho-phenol hydroxylation activity of PPO.