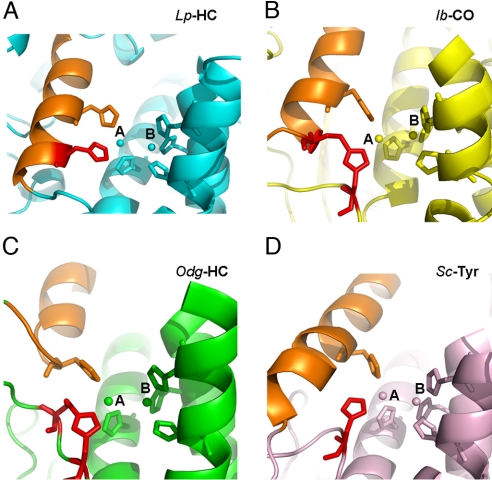
Fig. 3.
Different histidine coordination at CuA site. The active sites of 4 representative type 3 copper proteins are shown in the ribbons. Limulus polyphemus hemocyanin (Lp-HC, PDB ID code 1oxy), cyan (A); sweet potato Ipomoea batatas catechol oxidase (Ib-CO, PDB ID code 1bug), yellow (B); Octopus dofleini hemocyanin (Odg-HC, PDB ID code 1js8), green (C); and Streptomyces castaneoglobisporus tyrosinase (Sc-Tyr, PDB ID code 1wx5), salmon (D). The di-copper atoms and their 6-histidine ligands are shown in spheres and sticks, respectively. The unique histidine ligands at CuA sites are colored in red for comparison. The structurally conserved α-helix, where a second histidine locates, is colored in orange for reference in each structure. In Lp-HC, the histidine in comparison is located on the same α-helix where another histidine ligand resides. Notice that the thioether bond in Ib-CO is formed between 2 secondary structures, tethering the histidine residue at the CuA site, which resembles the CuA coordination in Lp-HC. In comparison, the equivalent histidine residues in both Odg-HC and Sc-Tyr are flexible. The thioether bond in Odg-HC is formed within the flexible loop, whereas that in Sc-Tyr does not form any thioether bonds.