Figure 1. Characterization of LoVo cells overexpressing wild-type (WT) PTHrP or PTHrP deleted over the nuclear localization signal (ΔNLS) or the signal peptide (ΔSP).
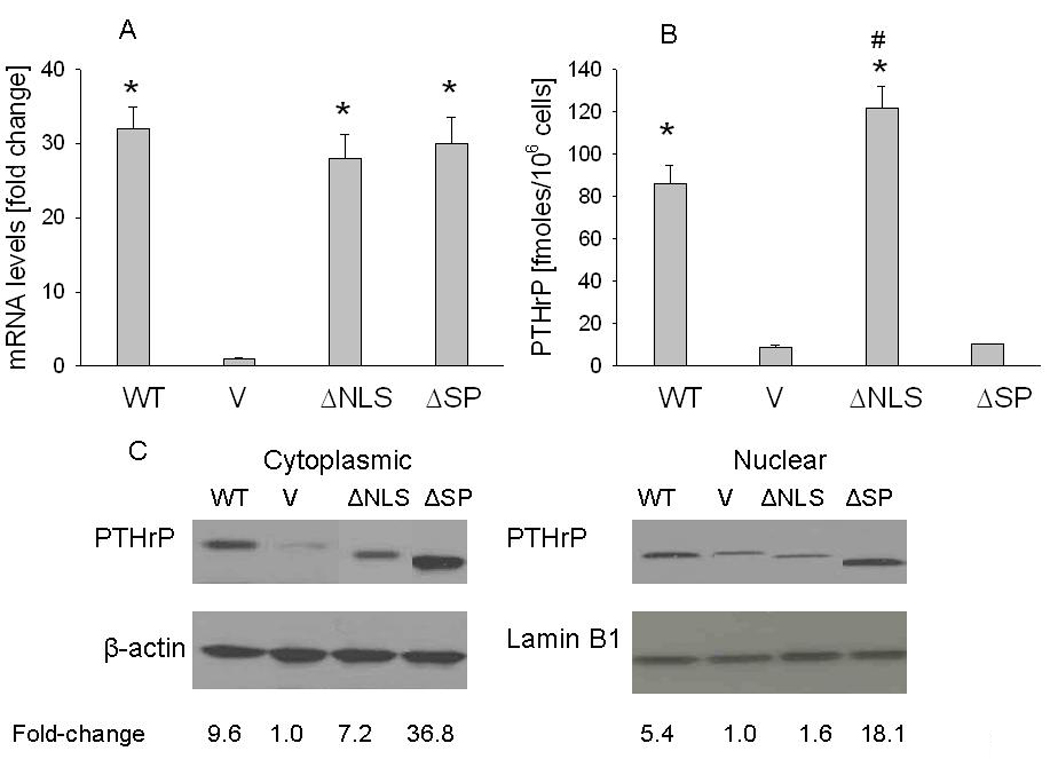
V = empty vector controls. (A) mRNA levels were measured by reverse transcription/real-time PCR. Values are expressed relative to the corresponding V value, set arbitrarily at 1.0. (B) PTHrP secretion was measured using an immunoradiometric assay. Values are expressed as PTHrP secreted in fmoles/106 cells. In (A) and (B), each bar is the mean ± SEM of three independent experiments for each of three independent clones. * = Significantly different from the control (V) value (P < 0.001); # = significantly different from the WT value (P < 0.001). (C) Western blot analysis of cytoplasmic and nuclear PTHrP fractions. WT PTHrP was detected as an ~ 18 kDa protein. Equal loading was confirmed by re-probing for cytoplasmic (β-actin) or nuclear (lamin B1) markers. The densitometric scanning data represents the mean fold-change obtained from two experiments for each of three independent clones. Values are expressed relative to the corresponding V value, set arbitrarily at 1.0.
