Abstract
High frequency stimulation (HFS) is used to control abnormal neuronal activity associated with movement, seizure, and psychiatric disorders. Yet, the mechanisms of its therapeutic action are not known. Although experimental results have shown that HFS suppresses somatic activity, other data has suggested that HFS could generate excitation of axons. Moreover it is unclear what effect the stimulation has on tissue surrounding the stimulation electrode. Electrophysiological and computational modeling literature suggests that HFS can drive axons at the stimulus frequency. Therefore, we tested the hypothesis that unlike cell bodies, axons are driven by pulse train HFS. This hypothesis was tested in fibers of the hippocampus both in-vivo and in-vitro. Our results indicate that although electrical stimulation could activate and drive axons at low frequencies (0.5 – 25 Hz), as the stimulus frequency increased, electrical stimulation failed to continuously excite axonal activity. Fiber tracts were unable to follow extracellular pulse trains above 50 Hz in-vitro and above 125 Hz in-vivo. The number of cycles required for failure was frequency dependent but independent of stimulus amplitude. A novel in-vitro preparation was developed, in which, the alveus was isolated from the remainder of the hippocampus slice. The isolated fiber tract was unable to follow pulse trains above 75 Hz. Reversible conduction block occurred at much higher stimulus amplitudes, with pulse train HFS (>150 Hz) preventing propagation through the site of stimulation. This study shows that pulse train HFS affects axonal activity by: (1) by disrupting HFS evoked excitation leading to partial conduction block of activity through the site of HFS; and (2) generating complete conduction block of secondary evoked activity, as HFS amplitude is increased. These results are relevant for the interpretation of the effects of HFS for the control of abnormal neural activity such as epilepsy and Parkinson's disease.
Keywords: white matter, deep brain stimulation, hippocampus, conduction block
INTRODUCTION
High frequency electrical stimulation (HFS) has been applied clinically to treat movement disorders such as Parkinson's disease and dystonia with deep brain stimulation (DBS) electrodes (Benabid et al. 1998; Hung et al. 2007). In addition, DBS therapies have been explored for a number of other neurological disorders including depression, obsessive compulsive disorder, and epilepsy (Abelson et al. 2005; Hardesty and Sackeim 2007; Velasco et al. 2001a; Velasco et al. 2001b). Prior research has shown that pulse train HFS suppresses somatic neural activity (Beurrier et al. 2001; Lian et al. 2003) but it is unclear what effect HFS has on axons surrounding the stimulation electrode (McIntyre et al. 2004a). Determining the effect of HFS on axons is critical to understanding how electrical stimulation controls neural activity in these diverse neurological disorders. Therefore, in this study, we tested the hypothesis that unlike cell bodies, axons are driven by continuous, pulse train HFS.
Although low frequency stimulation (LFS; orthodromic and antidromic) is commonly used to characterize hippocampal pathways and network dynamics (Leung 1979a; Meeks et al. 2005; Raastad and Shepherd 2003), the effect of extracellular HFS on neural elements remains controversial. Many studies investigating the effects of HFS have been conducted using preparations targeting cellular regions such as the basal ganglia (subthalamic nucleus, globus pallidus) (Beurrier et al. 2001; Garcia et al. 2003), thalamus (Houeto et al. 2005), and the hippocampus (Bikson et al. 2001; Lian et al. 2003). Based on these studies, HFS is thought to suppress somatic activity. However, none of these studies examined the effects of electrical stimulation on axons within the area of stimulation. In the current study, rat transverse hippocampal slices were used as the tri-synaptic pathway provides several potential fiber tracts in which to test the effects of pulse train stimulation.
While in-silico and in-vivo studies confirm HFS induced suppression of cell bodies, they provide contradictory data on whether HFS excites or inhibits the axons of projection neurons and afferent inputs (Dostrovsky and Lozano 2002; McIntyre et al. 2004b; Vitek 2002). Recent work has shown that sinusoidal HFS suppresses axonal conduction in-vitro (Jensen and Durand 2007b), while studies using pulse train HFS indicate a variety of differing effects on axonal conduction and synaptic efficacy (Iremonger et al. 2006; Schiller and Bankirer 2007). Differences in stimulus waveforms may be important to observed neural activity in response to stimulation; specifically, the steeper rise times associated with prolonged pulse train stimulation may produce an excitatory response rather than the suppressive response seen with sinusoidal waveforms. More recently, fiber tract stimulation is being investigated as a therapy for refractory psychiatric disorders (Abelson et al. 2005; Hardesty and Sackeim 2007; Mayberg et al. 2005). Despite its therapeutic promise, the use of direct fiber tract stimulation to control axonal conduction remains under-explored.
While short bursts of HFS (orthodromic and antidromic) have been used to generate cellular LTP or network oscillations (Martin and Shapiro 2000; Traub et al. 2004; Vreugdenhil et al. 2005), the effect of HFS on axonal conduction and action potential fidelity itself is unclear. Recent studies of axonal fidelity show few conduction failures using low frequency extracellular monophasic stimulation. As the stimulation frequency increases (above 100 Hz), decreased fidelity becomes more common (as much as 30-50% reduction in amplitude before failure) (Meeks et al. 2005; Raastad and Shepherd 2003; Soleng et al. 2004). In addition, axons can have reliable fidelity in response to intracellularly evoked action potentials (Meeks et al. 2005), but may behave differently when activated extracellularly, since the simultaneous activation of many axons can modify tissue excitability. In this study, we report that pulse train HFS is unable to drive axons for prolonged stimulus periods and generates reversible conduction block with increasing stimulus amplitude both in-vitro and in-vivo preparations. These data indicate that HFS of fiber tracts has the potential for controlling abnormal propagating activity within the brain without surgical resection of white matter tracts. Preliminary results have been reported in abstract form (Jensen and Durand 2007a).
METHODS
In-vitro hippocampal slice preparation
In-vitro experiments were performed in the alvear and CA1 regions of transverse hippocampal slices prepared from Sprague-Dawley rats (Zivic Miller). Rats were anesthetized using ethyl ether and decapitated. Experimental protocols were approved by the Institutional Animal Care and Usage Committee. The brain was removed from the skull and sectioned twice, caudally to detach the cerebellum and midsagitally to separate the two hemispheres. One hemisphere was glued (sagital face down) to the specimen disc of a vibrating-blade microtome (VT1000S, Leica, Nusslock, Germany) with cyanoacrylate, and secured in the microtome cutting chamber filled with ice-cold (3-4°C), oxygenated (O2 95%, CO2 5%), sucrose-rich artificial cerebrospinal fluid (ACSF), consisting of (mM): sucrose 220, KCl 3, NaH2PO4 1.25, MgSO4 2, NaHCO3 26, CaCl2 2, dextrose 10 (pH 7.45). The resulting hippocampal transverse slices (350 μm) were immediately immersed in oxygenated (O2 95%, CO2 5%), normal ACSF (nACSF) consisting of (mM): NaCl 124, KCl 3.75, KH2PO4 1.25, MgSO4 2, NaHCO3 26, CaCl2 2, dextrose 10 (pH 7.4), and incubated at room temperature for at least 60 min before being transferred to an interface-recording chamber (Harvard Apparatus, MA) (nACSF, Temperature = 33 ± 2°C, bubbled with O2 95%, CO2 5%). Slices were discarded 6-8 hrs post incubation. All experiments were conducted using nACSF, unless otherwise noted.
Extracellular field recordings were made using glass microelectrodes (3-10 MΩ) filled with 150mM NaCl. Antidromic, evoked potentials (AEP) and compound action potentials (CAP) were simultaneously recorded in the CA1 stratum pyramidale and alvear axon field of hippocampal slices (Figure 1). All signals were amplified using an Axoclamp-2A microelectrode amplifier (Axon Instruments, Inc., Union City, CA), low-pass filtered (5kHz field potentials) with additional amplification via a FLA-01 (Cygnus Technology, Delaware Water Gap, PA), and simultaneously stored on a DT-200 digital tape recorder (Microdata Instrument Inc., S. Plainfield, NJ), and on computer via an optical data acquisition program (44.1 kHz sampling, Audio Companion, Roni Music).
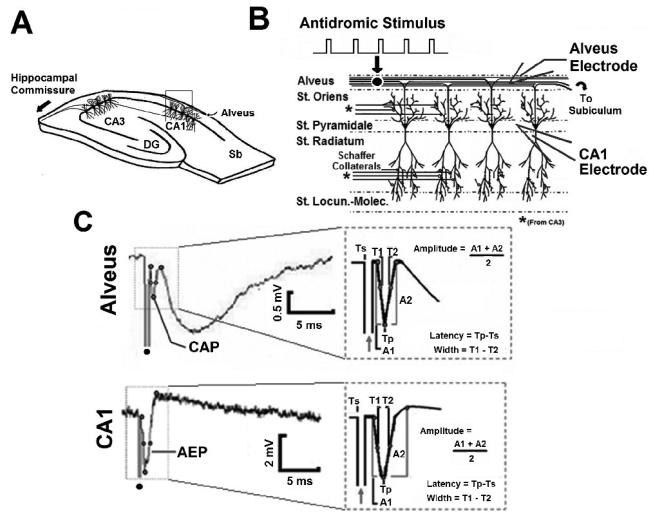
Fig. 1. Field potentials evoked by hippocampal fiber tract stimulation in-vitro.
A. In-vitro experimental schematic. Sb – Subiculum, DG- Dentate Gyrus. B. Extracellular field potentials were recorded simultaneously in the CA1 stratum pyramidale (CA1 electrode) and alveus (Alvear Electrode). C. Simultaneously recorded compound action potential (CAP) and antidromic evoked potential (AEP) in-vitro in response to antidromic stimulation of the alveus. Dot denotes stimulus artifact. Inset: AEP and CAP amplitude, width, and latency measurements.
Isolated Alvear Preparation
Using transverse hippocampal slices (350 μm), a novel preparation was developed, in which, the axon field of the alveus was isolated from the rest of the slice. Alvear dissection was achieved using sharp glass tools prepared in house. Visualizing the hippocampal slice through the microscope, a fine tipped brush was used to gently secure the hippocampal slice at the dentate gyrus. The sharp tip of a glass tool was used as a knife edge to carefully separate the alveus away from the stratum oriens. The remainder of the slice was discarded leaving only a thin strip of tissue containing the alveus (Figure 8). Extracellular field recordings were made in the isolated alveus using glass microelectrodes (as above). Tungsten electrodes were used to generate evoked and high frequency stimuli.
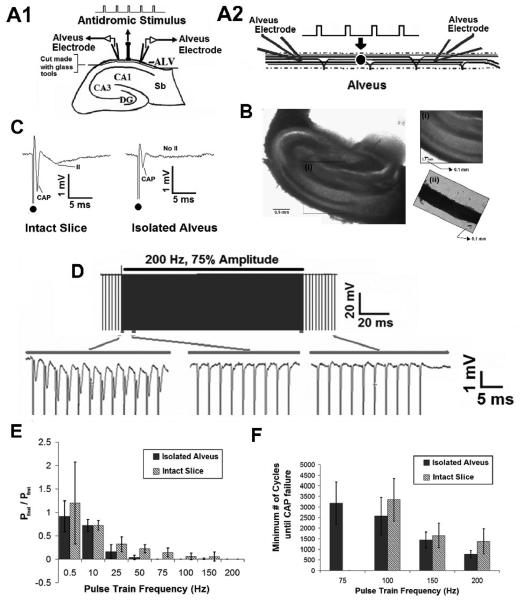
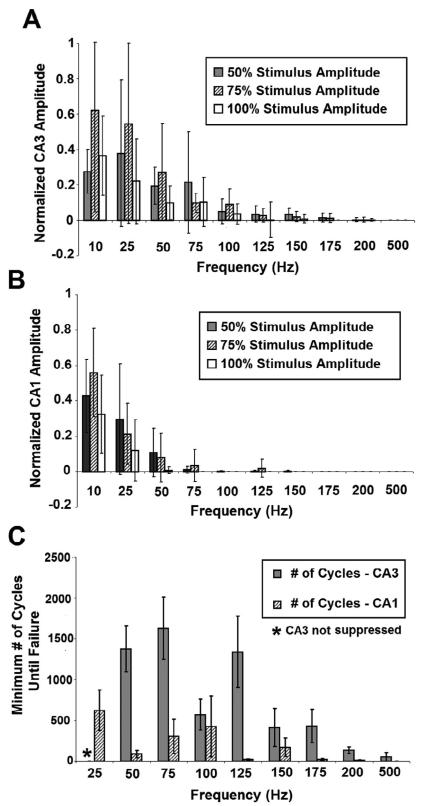
Fig. 8. Evoked Axonal Responses are unable to follow HFS in the Isolated Alveus.
A1 Transverse hippocampal slice. A2. Schematic of the isolated alvear network showing stimulating and recording electrode placement. B. Photograph of transverse hippocampal slice. (i) Inset: Magnification of the alvear axon field in the intact slice. (ii). Inset: Isolated alvear axon field. C. Example of the evoked alvear potential in the intact slice and isolated alveus. In the isolated preparation, the CAP is present but the secondary synaptic activation (II) is abolished, in response to an evoked stimulus. D. Evoked axonal activity (CAP) were unable to follow HFS even after isolation of the alvear axon field in-vitro (200Hz, 75% Amplitude). E. Failure to follow pulse train stimulation was frequency dependent. Changes in amplitude where analyzed by normalizing the amplitude of the final pulse in the train to the first pulse in the train (n=5). F. No statistically significant difference in the number of cycles until CAP failure between the intact slice and isolated alveus was observed using pulse trains above 75 Hz. In both cases, the number of cycles until CAP failure decreased as stimulus frequency increased (p>0.9, ANOVA, comparing isolated alveus and full slice).
In-vivo hippocampal preparation
Sprague-Dawley rats (300-450 g) were anesthetized with urethane (1.5 gm/kg, i.p.) and placed in a stereotaxic apparatus. Body temperature was maintained at 37 °C using a heating pad located under the animal. The skull over the left and right cortices was opened, and the dura removed. Warm ACSF (37 °C) was placed over the exposed cortical surface. The solution was refreshed every 5 minutes throughout the experiment by sucking away old solution and dropping in fresh solution using syringes (Feng and Durand 2003). Recording probe pairs were positioned in the left and right hippocampi to simultaneously record field potentials in both the CA3 and CA1 pyramidal layers. The recording probes were constructed from tungsten microelectrodes with a 0.8 mm vertical tip separation and 0.9 mm horizontal depth separation. A tungsten monopolar electrode was used to stimulate the ventral hippocampal commissural fibers (CF). The resulting evoked potentials were used to guide vertical positioning of the recording and stimulation electrodes (Kloosterman et al. 2001). Two stainless steel screws fixed in the bone of the nose and skull served as the ground and reference electrodes, respectively. Field signals were amplified 1000 times by two Model 1700 4-channel amplifiers (A-M System Inc.) with filter frequency ranges from 0.1 Hz to 5 KHz. Signals were then sampled at a rate of 20 KHz by using a ML795 PowerLab/16SP data acquisition system (ADInstruments) before they were stored onto a hard disk for off-line analysis.
Stimulation parameters
Monophasic cathodic pulses (100ms, 0.5-200 Hz,) were applied to the alveus of transverse hippocampal slices in-vitro or the commissural fiber pathway in-vivo. Pulse trains were applied at 100%, 75%, and 50% of the stimulation amplitude required to produce a maximal compound action potential. For the purposes of this study, high frequency stimulation (HFS) was defined as continuous, monophasic pulse trains with stimulus frequencies above 50 Hz. For paired pulse experiments, stimuli were applied using interpulse intervals (IPIs) of 2.5-100 ms (10-400 Hz). For conduction block experiments, two monopolar tungsten electrodes were used to independently deliver the test pulses (0.5 Hz) and pulse train HFS (100-200 Hz) to the fiber tract. In-vitro stimuli were generated using either a Grass S88 Stimulator or Wavetek Arbitrary Waveform Generator 395. In-vivo stimuli were controlled via computer interface (Chart v5.3, PowerLab amplifier). Voltage waveforms were converted to a current by stimulus isolator units (A-M system digital 2300 and analog 2200 isolators).
Data Analysis
Evoked potential amplitude, width, and latency were measured, as shown in Figure 1C. Measurement technique was the same for both in-vitro and in-vivo preparations. Amplitude (mV) was measured by taking the average of the falling and rising phases of the initial positive-negative-positive components of the evoked potential. Width (ms) was defined as the time between points located at 50% amplitude of the falling and raising phases of the evoked potential, while latency (ms) was the time from onset of evoked stimulus artifact to the first negative peak of the response. Field potential amplitude, width, and latency were analyzed prior, during, and post pulse train HFS. Unless noted otherwise, CAP and AEP morphology (amplitude, width, and latency) were normalized to pre-stimulus values in order to quantify the effect of pulse train HFS on evoked somatic and axonal activity. Extracellular field potentials did not require averaging before normalization using pre-stimulus values.
A paired pulse paradigm was used to assess the ability for fiber tracts to follow short duration stimuli, and investigate the role of refractory period on changes in axonal following. The amplitude, width, and latency of evoked response were measured for the first pulse (P1, W1, L1) and the second pulse (P2, W2, L2). The ratio of the amplitude (P2/P1), width (W2/W1), and latency (L2/L1) provided information about the effect of refractory period on evoked field responses.
Data was analyzed off-line using a combination of MATLAB (MathWorks Inc.), PowerLab Chart v5.3 (ADInstruments Inc.), and Minitab 15 (Minitab Inc.). Unless noted otherwise, all data are given as the mean ± SD, where n represents the number of slices, and N represents the number of evoked responses or traces. Paired, two tailed student's t test, and/or ANOVA (p = 0.05) were applied for statistical comparison.
RESULTS
Field potentials evoked by axonal stimulation in the hippocampus in-vitro and in-vivo
To characterize the activation of fiber pathways, monophasic, cathodic stimulation (0.5 Hz, 100 μs, 100% max threshold) was applied to either the alveus of transverse hippocampal slices in-vitro or the commissural fiber (CF) pathway in-vivo.
Extracellular field potentials were recorded simultaneously in the CA1 stratum pyramidale (CA1 Electrode) and alveus (Alveus Electrode) of transverse hippocampal slices in-vitro (Figure 1A). A single stimulus applied to the alveus generated an antidromic, evoked potential (AEP) recorded the CA1 cell layer, and a propagating compound action potential (CAP) recorded in the alveus (Figure 1B). The AEP was characterized by a negative population spike (N = 268 evoked responses); 3.5 +/− 1.7 mV amplitude, 0.8 +/− 0.1 ms width, with a peak at 1.7 +/− 0.3 ms) following the stimulus (bottom trace, Figure 1C). The evoked potential recorded in the alveus (top trace Figure 1C) had three overlapping components: (I) a brief, negative antidromic compound action potential (CAP) (N = 335 evoked responses); 0.6 +/− 0.3 mV amplitude, 0.7 +/− 0.2 ms width, with a peak at 1.5 +/− 0.2 ms); (II) a slow, negative potential associated with CA1 field events; and (III) a long-lasting, slow positive potential (not shown) (Leung 1979a; b). The characteristics of the in-vitro CAP are similar to those described by Leung for in-vivo alvear stimulation and recording (Leung 1979a; b). The in-vitro CAP and AEP have previously been characterized in detail using pharmacology (Jensen and Durand 2007b). In brief, bath application of 100μM Picrotoxin (PTX), 50μM D-(−)-2-amino-5 phosphonopentanoic acid (D-APV), and 40μM 6,7-dinitroquinoxaline-2,3-dione (DNQX) completely and reversibly eliminated the synaptic components (II, III) of the evoked alvear response leaving the CAP (component I) intact (Jensen and Durand 2007b). The AEP was not significantly affected by the bath applied receptor antagonist cocktail. Additionally, bath application of tetrodotoxin (500nM, TTX ) completely and reversibly blocked both the CAP and AEP (Jensen and Durand 2007b).
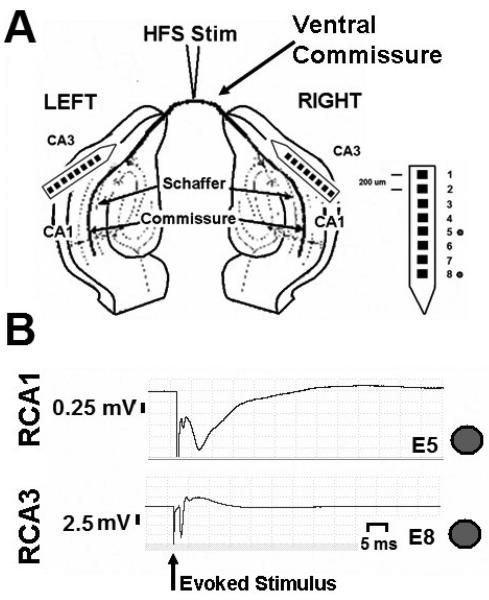
In response to commissural fiber (CF) stimulation, antidromic evoked field potentials were recorded in the CA3 region, while orthodromic field potentials were recorded in the CA1 region of both hippocampi in-vivo (Figure 2A). Amplitude, width and peak latency were measured in the same way as in-vitro evoked potentials (Figure 1C). The antidromic, evoked potential in CA3 (N = 156 evoked responses, Figure 2B bottom trace); 4.5 +/− 3.0 mV amplitude, 0.7 +/− 0.2 ms width, with a peak at 2.2 +/− 0.4 ms), and orthodromic, evoked potentials in CA1 (N = 100 evoked responses); 1.6 +/− 1.3 mV amplitude, 1.1 +/− 1.3 ms width, with a peak at 3.4 +/− 2.1 ms) were characterized by the presence of a negative population spike followed, in some cases, by slow field potentials in-vivo (Figure 2B top trace). The presence of slow, negative field potentials was more prominent in the CA1 region of the hippocampus than in CA3. The slow field potentials associated with CF evoked responses are the result of di- and poly-synaptic connections within the CA1 and CA3 hippocampal regions. Slight variations in the orthodromic CA1 and antidromic CA3 responses were observed depending upon the placement and depth of recording and stimulating electrodes in-vivo. Direct axonal recordings from the commissural fibers were not made in-vivo.
Fig. 2. Field potentials evoked by commissural fiber tract stimulation in-vivo.
A. Hippocampal potentials (CA1, CA3) were recorded bilaterally in response to commissural fiber stimulation in-vivo. B. Field potentials generated in response to commissural tract stimulation from right hippocampus are shown. Top trace, evoked field response from CA1. Bottom trace, evoked field response from CA3. Dot denotes stimulus artifact.
Long duration HFS does not drive prolonged evoked field responses
Current literature suggests that pulse trains drive axons at the stimulation frequency disrupting synchronous, abnormal activity in the network (Johnson and McIntyre 2008; McIntyre et al. 2004a). Yet, recent work has shown changes in axonal fidelity in response to high frequency stimulation (HFS) (Iremonger et al. 2006; Meeks et al. 2005), and suppression of axonal conduction in response to applied sinusoidal HFS (Jensen and Durand 2007b). To determine whether prolonged pulse train stimulation of a fiber tract drives axonal firing, monophasic extracellular stimulation (0.5-200 Hz, 100 μs, 1-2 min) was applied via a monopolar tungsten electrode to either the alveus of transverse hippocampal slices in-vitro (rat, n=5) or the commissural fiber pathway in-vivo (rat, n=7). Field potentials were recorded in the CA1 stratum pyramidale and alveus in-vitro or the CA1 and CA3 regions of both hippocampi in-vivo. Pulse trains were applied at 100%, 75%, and 50% of the stimulation amplitude producing a maximal evoked response.
Long duration pulse train HFS in-vitro
Contrary to our hypothesis, high frequency stimulation (HFS) was unable to drive synchronized axonal responses during prolonged pulse train HFS in-vitro. An example trace is shown in Figure 3A. At 0.5 Hz, evoked responses (antidromic, evoked potential (AEP) and compound action potential (CAP)) followed the alvear stimulation in a one-to-one manner. When continuous pulse train HFS was applied to the alveus, a brief period of HFS evoked excitation occurred, where the initial pulses of stimulation produced one to one following with approximately 20% of the response amplitude seen prior to HFS. Prolonged 200 Hz pulse train stimulation failed to drive axonal activation, resulting in the loss of extracellularly recorded field potentials (Figure 3A, inset, middle and end of stimulation). These results were observed with stimulation above 50 Hz (Figure 3B). Following HFS termination and return to 0.5 Hz stimulation, the evoked field potentials returned. However, the amplitude was diminished when compared to pre-HFS levels (0.55 ± 0.46, normalized amplitude, p < 0.0001, Student 2-sample-t test, N = 53). The sustained reduction in amplitude after termination of HFS indicates that axonal blockade is a possible mechanism underlying the failure of evoked responses to follow pulse train HFS. By 60 s following HFS termination, the evoked response had returned to pre-HFS amplitudes. Pulse train HFS effects recorded at the somatic layer (AEP) followed a similar pattern.
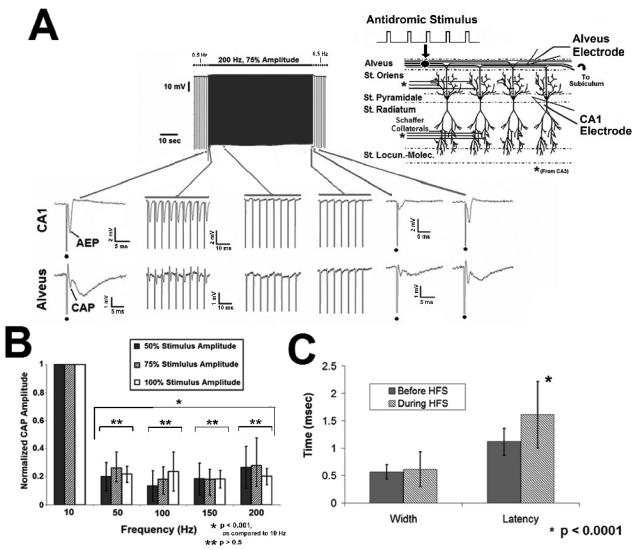
Fig. 3. Evoked axonal responses fail to follow HFS pulse trains in-vitro.
A. Evoked responses (CAP) failed to follow prolonged pulse train HFS. The frequency of the evoked stimulus given before and after HFS was 0.5 Hz. The example illustrates the effect of a 200 Hz pulse train. HFS generates initial excitation that decreases in amplitude over the stimulus duration, until the evoked response is gone. Dot denotes stimulus artifact during 0.5 Hz control stimulation. B. Failure of HFS evoked excitation is frequency dependent (p<0.001, ANOVA), with responses unable to faithfully follow pulse train HFS above 50 Hz. Effects on somatic activity (AEP) were similar (not shown). C. CAP width and latency increased prior to complete failure (mean +/− SD; p<0.016, p<0.0001, respectively, student's paired t test), AEP width and latency were unchanged (p<0.2, student's paired t test; not shown).
Prolonged stimulation produced marked reduction of the HFS evoked excitation amplitude above 50 Hz prior to complete failure (84 ± 8 %, (CAP); 87 ± 12 % (AEP)) (Figure 3B, p<0.001, across all frequencies, as compared to 10 Hz, ANOVA; AEP data not shown). There was no difference between HFS evoked excitation amplitude at 0.5 Hz and 10 Hz (p>0.5, Student's paired t test). The failure of HFS evoked activity to follow the stimulation was independent of stimulus amplitude over the range tested (50, 75, 100% threshold to evoked maximal CAP) (Figure 3B, p > 0.5, ANOVA; AEP data not shown). Similar stimulus mediated effects were observed in the stratum radiatum in response to pulse train activation by Schaffer Collateral HFS (data not shown). Differences in the width and latency of the CAP (axonal response) may indicate changes in conduction velocities across the alvear fiber population and possible desynchronization of the units underlying the field response. To determine whether desynchronization occurs in response to pulse train HFS, the width and latency of HFS evoked potentials within the alveus (CAP) prior to failure of those potentials to faithfully follow the stimulation was measured, and compared to evoked activity at baseline. Prior to complete CAP failure, the width and latency of HFS excitation increased during pulse train HFS (Figure 3C; width: p<0.016, student's paired t test; 0.51 ± 0.11 ms (control), 0.63 ± 0.30 ms (during HFS); latency: p<0.0001, student's paired t test; 1.12 ± 0.25 ms (control), 1.61 ± 0.61 ms (during HFS)). AEP width (1.27 ± 0.36 (control), 1.12 ± 0.54 (during HFS)) and latency (1.74 ± 0.6 (control), 2.08 ± 0.85 (during HFS)) were unchanged in response to HFS prior to complete suppression (p > 0.2, student's paired t test; data not shown). Additionally, the variability of the CAP width (HFS evoked excitation) increased during pulse train stimulation, as reflected in the standard deviation of the CAP width (p<0.0001, F- test, comparison of σbeforeHFS 2 and σduringHFS2). This “jitter” suggests greater variation in the timing of units underlying the HFS evoked response, across the population of fibers in the hippocampal alveus. In addition, the significant changes in CAP latency indicate that desynchronization could play a role in the initial loss of responses evoked by HFS at high stimulus frequencies. The minimum number of cycles required for complete failure of evoked potentials to follow pulse train stimulation was dependent upon stimulus frequency (p<0.001, as compared to 0.5 Hz control, ANOVA) and decreased as stimulus frequency increased (Figure 4). These data indicate that the duration of HFS will impact the observed effects of the stimulus upon network activity.
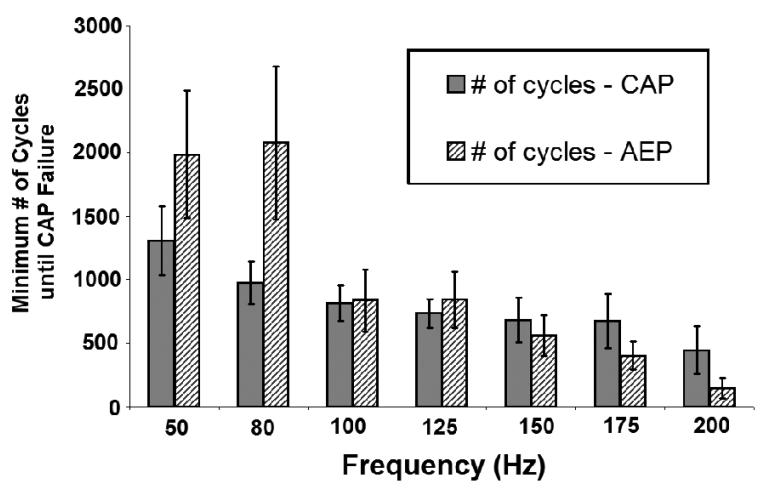
Fig. 4. Minimum number of pulse train cycles until CAP failure.
The minimum number of cycles until CAP failure decreases as HFS frequency increases, and was dependent on stimulus frequency but not stimulus amplitude (p<0.0001, p>0.5, respectively, ANOVA).
Long duration pulse train HFS in-vivo
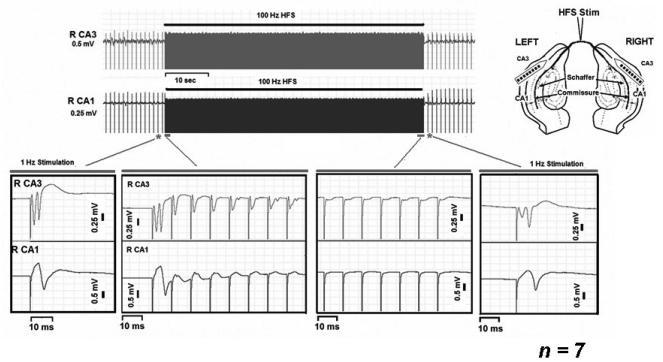
Low frequency (0.5-1 Hz) stimulation of the commissural fiber (CF) tract produced bilateral orthodromic and antidromic activation of the hippocampus in-vivo (Figure 5, inset, far left traces, right hemisphere). An example of CF pulse train HFS (100 Hz) is shown in Figure 5. Applied pulse trains generated an initial period of evoked excitation. With prolonged stimulation, evoked potentials failed to follow the CF stimulation in a one-to-one manner (Figure 5, inset: top trace CA3 evoked response, bottom trace CA1 evoked response). Loss of evoked field potentials persisted for the duration of HFS application. Evoked potentials returned following HFS termination, and returned to pre-HFS amplitudes by 2 minutes following HFS termination.
Fig. 5. Pulse train HFS of fiber tracts in-vivo.
The antidromic (CA3) and orthodromic (CA1) evoked responses failed to follow pulse train HFS of the commissural fiber tract (100 Hz). The frequency of the evoked stimulus given before and after HFS was 0.5 Hz. Inset, schematic of the in-vivo experimental arrangement.
The effect of stimulus frequency on antidromic (CA3) and orthodromic (CA1) excitation was assessed. As pulse train frequency increased, CA3 and CA1 response amplitude decreased by the final cycle of stimulation (Figures 6A, B; p<0.0001, ANOVA, as compared across frequencies above and below 125 Hz for 6A and 75 Hz for 6B). Normalized amplitude in-vivo was defined as the response amplitude produced by the final stimulus cycle divided by the amplitude of the control response prior to start of the pulse train. Antidromic activity (CA3; Figure 6A) was unable to follow pulse trains above 125 Hz, while orthodromic activity (CA1; Figure 6B) was unable to follow prolonged stimulation above 75 Hz. For stimulation frequencies between 10 and 75Hz, the variability in CF evoked response amplitude was large, with some reduction in amplitude observed during prolonged stimulation (Figure 6A, B). Using these frequencies, the variability in CF evoked response amplitude may be attributed to plastic changes at synapses within CA1 and CA3, as the field population was monitored (Malenka 1994). Additional studies are necessary to confirm this hypothesis. Direct CAP recordings from the commissural fibers were not achieved using the current in-vivo preparation.
Fig. 6. Failure to follow pulse trains in-vivo is frequency and amplitude dependent.
A, For antidromic activity (CA3), evoked activity decreased as frequency of stimulation was increased (p<0.0001, ANOVA, as compared across all stimulation frequencies), while stimulus amplitude had a slightly significant effect (p<0.017, CA3; ANOVA, as compared across all frequencies of stimulation). B. For orthodromic activity (CA1), evoked activity decreased as frequency of stimulation was increased (p<0.0001, ANOVA, as compared across all stimulation frequencies), while stimulus amplitude had a slightly significant effect (p<0.031, CA1; ANOVA, as compared across all frequencies of stimulation). C. The minimum number of pulse train cycles until complete failure was dependent on stimulus frequency for antidromic activation (CA3) (p<0.001, as compared across stimulation frequencies, ANOVA), but not orthodromic activation (p>0.1, as compared across stimulation frequencies, ANOVA).
For a given frequency, varying the stimulus amplitude did not impact the amount of failure observed in pulse train evoked antidromic and orthodromic responses (p<0.017, CA3; p<0.031, CA1; ANOVA, effect of amplitude compared across all stimulus frequencies). The minimum number of cycles until complete failure of the antidromic and orthodromic responses were dependent upon stimulus frequency (p<0.001 (CA3); p<0.031 (CA1), as compared across stimulation frequencies, ANOVA). As stimulus frequency increased, the number of cycles until failure decreased for both CA3 and CA1 activity (Figure 6C). Failure of the antidromic response was not observed below 50 Hz with CF HFS. In comparison, failure of orthodromic HFS evoked responses were observed using frequencies as low as 25 Hz.
Effect of Refractory Period on Axonal following in-vitro and in-vivo
Short bursts (2-20 pulses) of antidromic and orthodromic stimuli are commonly used to describe neural network function, axonal refractory period, and impulse fidelity (Kocsis et al. 1983; Meeks et al. 2005; Raastad and Shepherd 2003; Soleng et al. 2004). In the current study, axonal refractory period may limit the frequency at which the alvear fiber tract can respond to extracellular pulse train stimulation. Therefore, a paired pulse paradigm was used to investigate the effect of refractory period on axonal fidelity.
Paired pulse paradigm in-vitro
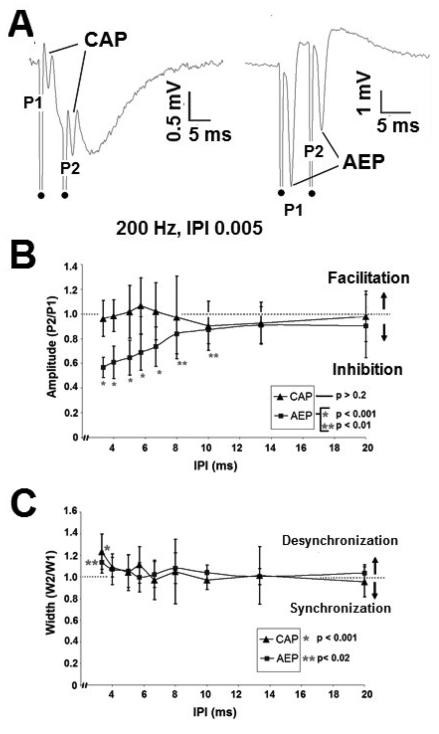
Stimuli were applied antidromically to the alveus using interpulse intervals (IPIs) of 2.5 to 100 ms (n=8, stimuli repeated 5 times per IPI at 75% of the threshold to evoke a maximum CAP for each slice). The amplitude, width, and latency of the evoked responses (CAP, AEP) were measured for the first (P1, W1, L1) and second pulses (P2, W2, L2). In the CA1 region, the CAP and AEP followed paired pulse stimuli up to 300 Hz. Above 300 Hz the stimulus artifact from the second pulse obscured the evoked response of the first. A typical paired pulse response (200Hz) is shown in Figure 7A. CAP amplitude was unaffected across IPIs tested (p>0.5, ANOVA, across IPIs, 2.5 to 100 ms); however, AEP amplitude showed significant inhibition (p<0.0001, ANOVA, Figure 7B). Below an IPI of 8 ms, the AEP amplitude ratio was significantly different than unity (P2/P1=1) (2 tailed, 1 sample t test, p < 0.001 (IPI 3.3ms – 6.6 ms)). Inhibition in the AEP (somatic response) is likely due to recurrent inhibition. Evoked response width and latency (CAP and AEP) were unaffected by the paired pulse paradigm (p>0.2, ANOVA, across IPIs, width - Figure 7C; latency not shown). These results indicate that refractory period is not the mechanism underlying failure of HFS evoked excitation to follow prolonged pulse train stimulation.
Fig. 7. Paired pulse paradigms in-vitro.
A. Paired pulse responses generated by 200 Hz (IPI 5 ms) applied to the alveus. Responses recorded extracellularly in the alveus (left) and CA1 pyramidal layer (right). Dot denotes stimulus artifact. B. Cellular field responses (AEP) demonstrated paired pulse inhibition above 100 Hz (IPI 10 ms), while alvear field responses (CAP) were unaffected by the paired pulse paradigm. Paired pulse amplitude ratio, (P2/P1). C. CAP and AEP width were unaffected by the paired pulse paradigm up to IPIs of 4 ms. Below an IPI of 4 ms, cellular (AEP) and axonal (CAP) paired pulse responses shown slight desynchronization. Paired pulse width ratio, (W2/W1).
Paired pulse paradigm in-vivo
Stimuli were applied to the ventral hippocampal commissural fibers (CF) using interpulse intervals (IPI) of 2.5 to 100 ms and amplitudes 50, 75, and 100% of the threshold to evoke a maximum response in CA3 (n=5, stimuli repeated 2 times per IPI at 50, 75, and 100% threshold for each animal). The amplitude, width, and latency of the evoked responses (CAP, AEP) were measured for the first (P1, W1, L1) and second pulses (P2, W2, L2). Tables 1 and 2 describe the effect of paired pulse stimulation of antidromic (CA3) and orthodromic (CA1) response in-vivo. Antidromic activity followed stimulation up to 300 Hz (IPI 3.33 ms) without loss of the first evoked response in the artifact of the second pulse. As IPI decreased the antidromic paired pulse response decreased slightly (Table 1). This decrease is likely due to recurrent inhibitory circuits in CA3. Below an IPI of 5 ms, the amplitude ratio was significantly different than unity (P2/P1=1) (2 tailed, 1 sample t test, p < 0.0001). Response width and latency were unaffected by interpulse interval (p > 0.2, p > 0.05, respectively, ANOVA, across all IPIs, Table 1). Orthodromic amplitude, width, and latency showed significant reduction in response to the paired pulse paradigm (Table 2, p<0.001, ANOVA, across all IPIs, Table 1). Inhibition of the orthodromic response is likely dependent upon vesicle depletion in-vivo. These data support the observations made in-vitro in response to paired pulse stimulation.
Table 1.
Antidromic paired pulse response in-vivo
| Stimulus IPI (ms) |
CA3 Paired Pulse Response |
||
|---|---|---|---|
| Amplitude | Width | Latency | |
| 3.33 | 0.80 ± 0.21 | 1.12 ± 0.17 | 1.03 ± 0.04 |
| 5 | 0.94 ± 0.53 | 1.11 ± 0.15 | 1.01 ± 0.14 |
| 6.67 | 0.91 ± 0.32 | 1.05 ± 0.16 | 1.03 ± 0.05 |
| 10 | 0.86 ± 0.28 | 1.09 ± 0.18 | 1.02 ±0.06 |
| 20 | 0.89 ± 0.35 | 1.05 ± 0.15 | 1.01 ± 0.05 |
| 40 | 1.13 ± 0.26 | 1.00 ± 0.11 | 0.99 ± 0.03 |
| 100 | 1.14 ± 0.23 | 1.00 ± 0.00 | 1.00 ± 0.00 |
Table 2.
Orthodromic paired pulse response in-vivo
| Stimulus IPI (ms) |
CA1 Paired Pulse Response |
||
|---|---|---|---|
| Amplitude | Width | Latency | |
| 3.33 | 0.32 ± 0.21 | 0.57 ± 0.69 | 0.44 ± 0.51 |
| 5 | 0.57 ± 0.53 | 1.03 ± 0.84 | 0.75 ± 0.48 |
| 6.67 | 0.44 ± 0.32 | 0.61 ± 0.77 | 0.47 ± 0.51 |
| 10 | 0.41 ± 0.28 | 0.65 ± 0.65 | 0.57 ± 0.52 |
| 20 | 0.52 ± 0.35 | 0.87 ± 0.57 | 0.80 ± 0.47 |
| 40 | 0.78 ± 0.26 | 1.20 ± 0 39 | 0.99 ± 0 24 |
| 100 | 1.01 ± 0.23 | 1.00 ± 0.00 | 1.00 ±0.02 |
Failure of evoked responses to follow HFS in the isolated of the alveus
To investigate the role of stratum oriens and stratum pyramidale in the failure of alvear axons to follow prolonged pulse train HFS, the alvear axon field was isolated, using a novel in-vitro preparation (Figure 8C). Pulse train stimulation (0.5 – 200 Hz) was applied to alveus of an intact transverse hippocampal slice (Figure 8A1), then the alvear axon field was isolated from the slice and the stimulus protocol was repeated (Figure 8A2) (n = 5). A single tungsten electrode was used to generate evoked alvear responses. Comparing the evoked, alvear response from the intact slice and isolated tract shows preservation of the compound action potential (CAP), while synaptic components (II) of the response are abolished (Figure 8B). The CAP morphology was similar to that previously published for pharmacological isolation of the CAP (Jensen and Durand 2007b). Response amplitude was normalized by dividing the amplitude of the final pulse by the amplitude of the first pulse in the train.
In the isolated alveus, there is an initial period of HFS evoked excitation before failure of evoked responses to follow the pulse train (Figure 8D). In the example, the CAP briefly follows 200 Hz pulse train HFS before loss of the field potential occurs (Figure 8D). HFS evoked excitation failure was stimulus frequency dependent (Figure 8E), with failure of evoked field responses occurring with stimulation as low as 75 Hz. In the intact slice, complete failure of evoked responses occurred at a higher stimulus frequency (100 Hz) (Figure 8E). As in the intact slice, the number of pulse train cycles required for failure in the isolated alveus decreased as stimulus frequency increased, with no significant difference in the number of cycles until failure between the intact slice and isolated alveus above 75 Hz (p>0.9, ANOVA, Figure 8F). These data suggest that failure of evoked response to follow HFS is independent of neural elements in the stratum oriens, and that these effects are non-synaptic in nature.
Propagation through the site of HFS: Conduction block versus failure to follow stimulation
A possible explanation for the failure of HFS evoked excitation to follow the stimulus could be desynchronization of the evoked activity at the site of stimulation rather than “true” conduction block in the tissue around the stimulation electrode. Therefore, to provide direct evidence of conduction block, a test pulse was sent through the site of stimulation in-vitro (n= 8, Figure 9). The placement of stimulating and recording electrode relative to CA1 neural elements is shown in the upper portion of Figure 9.
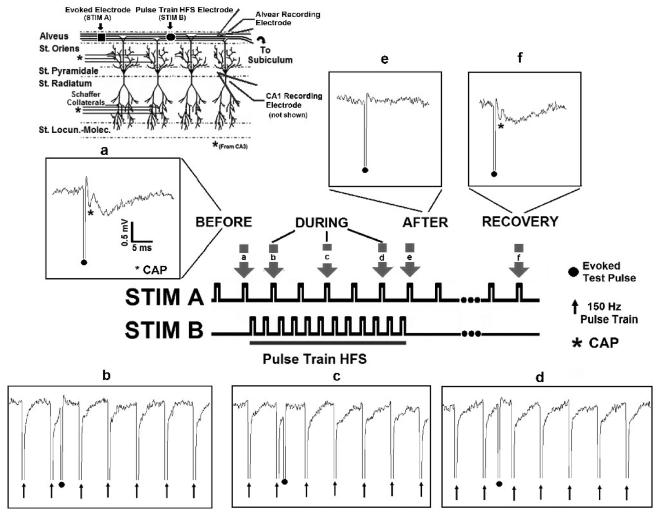
Fig. 9. Pulse train HFS blocks axonal conduction in-vitro.
Evoked test pulses were generated by a stimulating electrode placed in the alvear axon field. An independent electrode, located in the alveus, applied the pulse train HFS. Field potentials were recorded extracellularly in the alveus and CA1 somatic layer (see inset describing hippocampal network). (a) A robust evoked response (CAP, denoted by asterisk) was generated by STIM A (0.5 Hz) before HFS. Pulse train HFS (STIM B, 500 μA, 150Hz) blocks axonal conduction. Insets, b through d (evoked response, STIM A,). (e, f) Evoked responses (CAP) generated by STIM A are depressed following termination of HFS, but recover over ~ 60 seconds following HFS termination (n=8).
At pulse train frequencies of 150 – 200 Hz, HFS was capable of completely blocking the test pulse (STIM A) from passing through the site of stimulation (STIM B), as shown in Figure 9. Before pulse train application, a compound action potential (CAP) (denoted by asterisk) is generated by alvear stimulation (a, STIM A). Pulse train stimulation (150 Hz, 500 μA, STIM B) generates complete block of axonal conduction through the site of stimulation, as shown in insets b through d. Following pulse train termination, the CAP generated by the test stimulus is depressed (e). CAP amplitude returns to pre-stimulus levels ~ 60 seconds following HFS termination (f). Preliminary experiments in-vivo support these findings (data not shown).
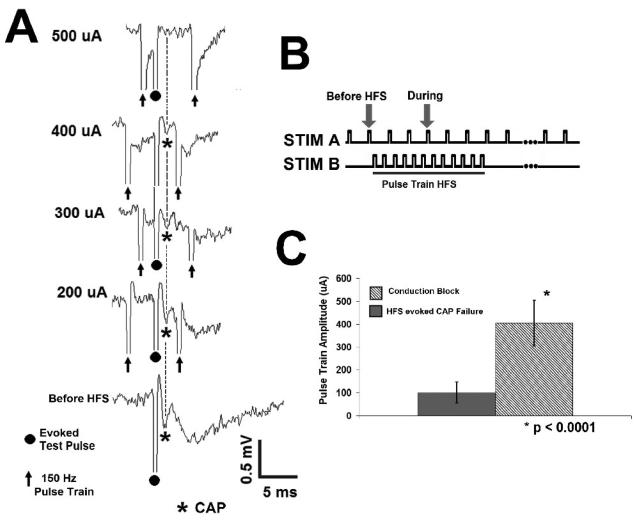
Conduction block is HFS amplitude dependent, as shown in Figure 10A. A representative evoked test pulse (CAP) is shown during applied 150 Hz pulse train stimulation (Figure 10A). As the HFS amplitude increased, CAP amplitude decreased until complete block was achieved (Figure 10A). The threshold current for complete block was 405 ± 101 μA (N= 17). At HFS subthreshold for complete block, the amplitude of the CAP traveling through the site of stimulation was 62 ± 44 % of pre-HFS amplitude. The width (0.5 ± 0.2 ms) and latency (1.45 ± 0.3 ms) of the evoked test pulse (CAP) does not change significantly subthreshold to complete block (p > 0.1, p > 0.05, respectively; Student's paired t test, as compared to pre-HFS values). In addition, the threshold for complete block is significantly larger than the HFS amplitude at which evoked potentials fail to follow pulse train HFS (405 ± 101 μA, 101 ± 46 μA, respectively, p<0.0001, Student's 2 sample t test, Figure 10C). Taken together, these data indicate that pulse train HFS is capable of generating axonal conduction block.
Fig. 10. Pulse train HFS Conduction Block is Amplitude Dependent.
A. Conduction block is amplitude dependent, with complete block occurring at higher stimulus amplitudes. B. Stimulation paradigm in-vitro. C. The threshold current for complete block is significantly larger than the stimulus amplitude at which evoked responses fail to follow pulse train HFS (405 ± 101 μA, 101 ± 46 μA, respectively, p<0.0001, Student's 2 sample t test).
How, then, do high frequency pulse trains affect axonal conduction? At HFS onset, the stimulus itself evokes activity. During prolonged stimulation at high stimulus frequencies, HFS evoked excitation is “suppressed”. At lower stimulus amplitudes, loss of HFS evoked excitation results from partial conduction block of the activity evoked by the stimulus itself within the axon field. At HFS amplitudes subthreshold to complete block, sending a test pulse through the site of stimulation indicates partial block, as does the sustained reduction in amplitude of the evoked response following HFS termination. As HFS amplitude increases, the test pulse amplitude is diminished until complete block is achieved.
DISCUSSION
Although high frequency electrical stimulation (HFS) is used as a therapy for several neurological disorders, controversy surrounds the direct action of this stimulation on different brain regions. Many HFS studies indicate suppression of target nuclei by the stimulation (Lian et al. 2003), while others indicate an increase in neural excitation (Boraud et al. 1996; Schiller and Bankirer 2007). Although the effects on adjacent fiber tracts are usually drawn by inference (Bar-Gad et al. 2004; Hashimoto et al. 2003), recent work in the hippocampus using sinusoidal high frequency electrical stimulation shows suppression of axonal conduction through extracellular axonal recordings in-vitro (Jensen and Durand 2007b). Of note, recent studies on STN-DBS indicate that axonal modulation seems to underlie the therapeutic efficacy of HFS (Gradinaru et al. 2009; Li et al. 2007). Therefore, direct stimulation of axonal tracts, as opposed to somatic nuclei, may provide a novel method for the control of neurological disorders. In line with this idea, white matter tract HFS is being considered for treatment of refractory psychiatric disorders (Johansen-Berg et al. 2007; Mayberg et al. 2005). This study investigates the ability of pulse train electrical stimulation to control axonal conduction. The data in this study show that (1) loss of HFS evoked responses occurs during prolonged stimulation in-vivo and in-vitro, (2) apparent failure of the HFS evoked excitation to follow low amplitude stimulation could indicate local desynchronization of stimulus evoked activity at the site of stimulation, and (3) pulse train HFS is capable of generating an amplitude dependent, reversible conduction block of propagating potentials.
In the current study, axons followed low frequency stimulation (0.5 – 10 Hz) in-vitro and in-vivo, with an evoked response being generated for every pulse. Pulse train evoked excitation was observed at the beginning of stimulation regardless of frequency, and is similar to previously reported activity (Bar-Gad et al. 2004; Hashimoto et al. 2003; Kimura and Pavlides 2000; Lee et al. 2005; Leung 1979a). During stimulus onset, HFS evoked excitation experienced an initial enhancement of the slow wave components of the alvear response, similar to that previously reported by Mori et al in response to short duration pulse trains (Mori et al. 2004). In the current study, the slow field components are present at the initial onset of the pulse train but are completely suppressed within 5-20 pulses (frequency >50 Hz). The presence or absence of slow field components are unlikely to contaminate the measurement of the CAP during pulse trains, as previously shown during sinusoidal HFS induced conduction block (Jensen and Durand 2007b). Additionally, pharmacological blockade of these components does not influence the presence nor measurement of the alvear CAP (Jensen and Durand 2007b). Approximately 50-100 ms into HFS application, HFS evoked response amplitude (CAP) began to diminish and continued to decrease with each stimulus cycle until the field response was gone. The loss of HFS evoked excitation was stimulus frequency dependent. Sustained reduction in the CAP following HFS termination suggests the presence of partial axonal block.
The stimulus parameters used in this study to generate failure of HFS evoked excitation to follow the stimulation and subsequent axonal conduction block are similar to those used in other HFS studies (Anderson et al. 2006; Iremonger et al. 2006; Lian et al. 2003). In the current study, HFS evoked excitation failed to follow stimulus frequencies above 50 Hz in-vitro and 125 Hz in-vivo, and are similar to stimulus frequencies associated with favorable deep brain stimulation (DBS) outcomes (~30Hz-300Hz) (O'Suilleabhain et al. 2003; Rizzone et al. 2001). Although other studies describe failure of axons to propagate faithfully in response to high frequency epilpetiform bursts (Meeks et al. 2005) and applied high frequency stimulation (~125-185 Hz) (Iremonger et al. 2006), some theoretical (McIntyre et al. 2004a; Miocinovic et al. 2006) and experimental (Hashimoto et al. 2003) evidence indicates that HFS has an excitatory effect on axons surrounding the stimulus electrode. A reason for these apparent differences in activation could be the effective stimulus amplitudes seen by the surrounding tissue (Anderson et al. 2006; Butson et al. 2006; Butson and McIntyre 2005). The complexity and heterogeneity of the brain regions stimulated can make it challenging to assess the overall outcome of HFS. As a result, it is unclear whether excitation, inhibition, or a balanced combination of both is indicative of therapeutic stimulation. Recent work provides strong evidence that modulation of afferent axons via axonal excitation (Bar-Gad et al. 2004; Hashimoto et al. 2003; Li et al. 2007) and/or axonal silencing (Gradinaru et al. 2009; Iremonger et al. 2006; Jensen and Durand 2007b) underlie the therapeutic effects of HFS. However, additional studies employing multi-site, multi-unit recording may be necessary to clarify the role of excitation and inhibition in therapeutic stimulation. Additionally, care should be taken when comparing electrical stimulation studies, as differences in electrode configuration will impact the threshold current needed to elicit specific neural responses (Butson and McIntyre 2006; Butson et al. 2006; Butson and McIntyre 2005). Finally, stimulus waveform could impact the threshold for HFS mediate effects. Comparing the data in this study to prior work in the hippocampus, the threshold for suppression of both somatic and axonal activity is lower with sinusoidal HFS than pulse train HFS (Jensen and Durand 2007b; Lian et al. 2003). In the case of pulse train HFS, the steeper characteristics of the pulses compared to the gradual rise time of the sinusoidal waveform may reduce its efficacy in generating conduction block.
The results of the current study suggest that the shorter the pulse train delivered to the tissue, the more likely the axon will follow the stimulus. For longer duration pulse trains, such as those used in therapeutic electrical stimulation, the evoked axonal response will only follow stimulation faithfully for several hundred cycles. The current study suggests that HFS evoked excitation may desynchronize during prolonged stimulation leading to the illusion of conduction block. As result, the more stimulus cycles applied during HFS, the less likely synchronized axonal activation is reaching downstream and upstream neural populations. This change in the axonal response may impact activation at downstream synapses, as well as disrupt ongoing activity within the somas of afferent populations. Failure of evoked response to follow stimuli one for one does not necessarily indicate the presence of conduction block (Campbell and Woo 1966; Casey and Blick 1969; Iremonger et al. 2006). To test for complete conduction block, an independent electrode should be used to generate a propagating response through the site of stimulation. In the current study, pulse train HFS reversibly blocked axonal conduction in an amplitude dependent manner. Subthreshold to complete block, a test pulse propagates through the site of stimulation with reduced amplitude. As HFS amplitude is increased, the amplitude of the propagating response diminishes until complete conduction block occurs. In current literature, high frequency stimulation of fiber tracts is thought to reduce the frequency at which axons are able to follow extracellular stimulation (Anderson et al. 2006; Iremonger et al. 2006; Schiller and Bankirer 2007). In those studies, these effects have been attributed to both desynchronization and partial axonal block; however, complete conduction block was not reported in these studies.
Prior studies investigating the effects of electrical stimulation typically have used a single electrode to deliver both the HFS and test stimuli. In light of the current results, this experimental paradigm is called into question. In the current study, the data suggest that HFS evoked excitation may desynchronize during prolonged stimulation. Desynchronization of field responses could lead to erroneous conclusions regarding the presence or absence of block, as well as the minimum threshold current required for these effects. Therefore, it is important for a test pulse to be sent though the site of stimulation to confirm or refute true conduction block. As result, the relative absence of pulse train mediated conduction block in HFS literature from the central nervous system is two-fold: (1) the stimulus amplitude was likely subthreshold to this effect, and (2) the lack of a secondary stimulating electrode to provide direct evidence of blockade. In the case of this study, changes in CAP width and latency suggest desynchronization of HFS evoked excitation at low stimulus amplitudes; yet, the prolonged reduction in CAP amplitude following HFS termination indicate the presence of conduction block. Using a test pulse sent through the site of HFS, complete, reversible conduction block was confirmed.
A potential limitation of this study is the use of monophasic high frequency stimulation. In general, clinical deep brain stimulation uses biphasic HFS to minimize the possibility of tissue damage at the site of stimulation (Grill and Mortimer 1995; Merrill et al. 2005). In the current study, the suppressive effects of monophasic stimulation on axonal and cellular activity were reversible, ruling out permanent damage at the site of stimulation. However, the use of biphasic stimulation may produce different effects upon the stimulated neural networks in-vitro and in-vivo. To investigate this possibility, biphasic HFS was applied to the alveus in-vitro. Biphasic pulse train HFS suppressed axonal and cellular activity in a manner similar to that observed with monophasic HFS (data not shown). Additionally, recent work using biphasic high frequency stimulation have reported results similar to those observed in the current study (Shin et al. 2007; Shin and Carlen 2008). Further experiments are being conducted in-vivo to explore this issue.
Mechanisms underlying conduction block by pulse trains
The results of this study indicate that pulse train HFS affects axonal conduction in multiple ways depending on stimulus parameters. The mechanisms underlying these effects could be explained by (1) influence of the axonal refractory period on HFS evoked excitation; (2) desynchronization of the underlying units of the compound action potential (CAP); (3) an increase in the threshold for HFS evoked excitation; or (4) suppression of activity at the site of stimulation via potassium mediated depolarization blockade.
Pulse trains of limited duration are routinely used to characterize network interactions (Martin and Shapiro 2000; Traub et al. 2004; Vreugdenhil et al. 2005). As stimulus frequency increases, the typical duration of these stimuli decreases, with most high frequency trains being milliseconds in duration. The paired pulse paradigm used in this study shows that hippocampal axons can follow a few stimuli over a wide range of frequencies (10-500 Hz, Figure 7). The maximal frequency explored has an interpulse interval of 2 ms (500 Hz). Even at this frequency, the CAP amplitude, width, and latency remained close to unity, indicating that the refractory period of the axons is less than 2 ms. As a result, the loss of evoked responses during long duration pulse trains is unlikely to be the result of axonal refractory period interfering with activation.
Upon onset, HFS generates a period of initial activity evoked in response to the stimulus. The HFS evoked response amplitude decreases over time, until it is completely gone. Yet, monitoring only response amplitude can lead to erroneous conclusions regarding the presence or absence of the activity. Desynchronization of the underlying units (action potentials) of the response can lead to a decrease in amplitude without actual suppression of the response (Campbell and Woo 1966; Casey and Blick 1969), and is reflected by an increase in both the width and latency of the evoked response (Bhadra and Kilgore 2004; Campbell and Woo 1966; Casey and Blick 1969). In this study, changes in CAP width and latency suggest that desynchronization could play a role in the loss of responses evoked by pulse trains at high frequencies (Figure 3). To investigate this possibility, a test pulse was sent through the site of stimulation. In contrast to the HFS evoked excitation, the secondary test pulse width and latency was unaffected by the pulse train prior to complete conduction block (Figure 10).
Pulse train HFS generated amplitude dependent, reversible conduction block. The HFS threshold for complete block was 3.2 ± 1.5 times the amplitude needed to evoke a maximal CAP. At HFS amplitudes immediately subthreshold to complete block, test pulses propagated through the site of stimulation with reduced amplitude, but no change in width or latency. These data indicate the presence of partial axonal block, rather than desynchronization of the test pulse or increased threshold of excitation. Partial conduction block could be caused by progressive inactivation of the sodium channel population in repetitive stimulation (De Col et al. 2008). The sustained reduction of the evoked response following HFS termination could also be explained by dependent sodium channel inactivation. Additionally, failure of HFS evoked excitation to follow the stimulus occurred at a much lower threshold than conduction block of the test pulse. At stimulus parameters threshold to failure of HFS evoked excitation, test pulses pass through the site of stimulation without significant changes to amplitude, width, or latency. Taken together, these data indicate that threshold of excitation is not significantly altered during HFS application.
In this study, pulse train HFS can generate conduction block. Electrical stimulation is known to generate stimulus frequency dependent changes in axonal fidelity (De Col et al. 2008; Iremonger et al. 2006; Meeks et al. 2005; Raastad and Shepherd 2003; Soleng et al. 2004) as well as elevations in extracellular potassium (Bikson et al. 2001; Jensen and Durand 2007b; Lian et al. 2003; Meeks et al. 2005; Shin et al. 2007). These stimulation mediated effects have been correlated with suppression of both somatic and axonal activity, as observed by loss of both field and intracellular potentials in different studies (Bikson et al. 2001; Iremonger et al. 2006; Jensen and Durand 2007b; Lian et al. 2003; Meeks et al. 2005; Meeks and Mennerick 2004). Therefore, simultaneous activation of a fiber tract by extracellular stimulation or even spontaneous activity could suppress axons leading to conduction block. Recent work by Meeks et al. using patch clamped axons supports this conclusion. Spontaneous epileptiform activity containing bursts of action potentials (20 Hz or greater) failed to propagate along axons in the Schaffer collateral region of the hippocampus (Meeks et al. 2005). Conduction block in axons may indicate a potential protective mechanism within the hippocampus, and elsewhere, where excessive, synchronous neural discharges involving a large number of axonal fibers are unable to propagate.
Pulse train stimulation has been associated with stimulus induced increases in extracellular potassium in the cortex (Heinemann and Lux 1977), the thalamus (Gutnick et al. 1979), the hippocampus (Hablitz and Lundervold 1981; Poolos et al. 1987), and the cerebellum (Kocsis et al. 1983). Additionally, elevated extracellular potassium concentrations are known to reduce action potential amplitude, depress presynaptic potentials, and affect axonal signaling (Hablitz and Lundervold 1981; Meeks et al. 2005; Meeks and Mennerick 2004; Shin et al. 2007). Potassium levels sufficient to produce changes in axonal and somatic activity have been recorded in response to sinusoidal HFS (Jensen and Durand 2007b; Lian et al. 2003) and pulse trains (Heinemann and Lux 1977; Lian et al. 2003) under pathological and physiological conditions. Therefore, a potential mechanism for the stimulus induced depression of axonal activity, then subsequent conduction block could be potassium mediated depolarization blockade (Beurrier et al. 2001). In support of this idea, recent computational models have suggested a role from potassium in stimulus mediated suppression of axonal activity through accumulation of potassium in the periaxonal spaces (Bellinger et al. 2008).
Clinical Implications
Axonal conduction block using high frequency stimulation (HFS) has the potential for controlling abnormal propagating activity within the brain without surgical resection of white matter tracts. Fiber tract stimulation would be an innovative method for control of abnormal neural activity in the brain and potentially applicable to a wide range of neurological disorder. However, it is unclear from the current literature if the stimulus parameters used in therapeutic stimulation would be associated with conduction block (Butson and McIntyre 2005; Kuncel and Grill 2004; Rizzone et al. 2001). The current study shows that stimulus duration impacts the neuronal effects observed in response to pulse train stimulation. Short duration trains generate time locked following of the evoked potentials, while longer duration stimulation generates axonal conduction block depending on stimulus amplitude. Only by comparing stimulus parameters across studies can a clearer view of the effects of high frequency stimulation on neural networks be established.
ACKNOWLEGMENTS
Special thanks to David Tang for his surgical expertise and assistance during the in-vivo experiments.
GRANTS
This work has been supported by NIH Grant R01-NS-40894, NIH Fellowship F31- NS-054416, a Fellowship from the Epilepsy Foundation, and Department of Education GAANN Neural Engineering Training Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abelson J, Curtis G, Sagher O, Albucher R, Harrigan M, Taylor S, Martis B, Giordani B. Deep brain stimulation for refractory obsessive-compulsive disorder. Biological Psychiatry. 2005;57:510–516. doi: 10.1016/j.biopsych.2004.11.042. [DOI] [PubMed] [Google Scholar]
- Anderson TR, Hu B, Iremonger K, Kiss ZHT. Selective Attenuation of Afferent Synaptic Transmission as a Mechanism of Thalamic Deep Brain Stimulation-Induced Tremor Arrest. J Neurosci. 2006;26:841–850. doi: 10.1523/JNEUROSCI.3523-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex Locking Rather Than Complete Cessation of Neuronal Activity in the Globus Pallidus of a 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Treated Primate in Response to Pallidal Microstimulation. J Neurosci. 2004;24:7410–7419. doi: 10.1523/JNEUROSCI.1691-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger S, Miyazawa A, Steinmetz P. Submyelin potassium accumulation may functionally block subsets of local axons during deep brain stimulation: a modeling study. J Neural Engineering. 2008;5:263–274. doi: 10.1088/1741-2560/5/3/001. [DOI] [PubMed] [Google Scholar]
- Benabid A, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollak P. Long-term electrical inhibition of deep brain targets in movement disorders. Movement Disorders. 1998;13:119–125. doi: 10.1002/mds.870131321. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Bioulac B, Audin J, Hammond C. High-Frequency Stimulation Produces a Transient Blockade of Voltage-Gated Currents in Subthalamic Neurons. J Neurophysiol. 2001;85:1351–1356. doi: 10.1152/jn.2001.85.4.1351. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Kilgore K. Direct current electrical conduction block of peripheral nerve. IEEE Trans Neural Syst Rehabil Eng. 2004;12:313–324. doi: 10.1109/TNSRE.2004.834205. [DOI] [PubMed] [Google Scholar]
- Bikson M, Lian J, Hahn PJ, Stacey WC, Sciortino C, Durand DM. Suppression of epileptiform activity by high frequency sinusoidal fields in rat hippocampal slices. J Physiol (Lond) 2001;531:181–191. doi: 10.1111/j.1469-7793.2001.0181j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Bioulac B, Gross C. High frequency stimulation of the internal Globus Pallidus (GPi) simultaneously improves parkinsonian symptoms and reduces the firing frequency of GPi neurons in the MPTP-treated monkey. Neuroscience Letters. 1996;215:17–20. doi: 10.1016/s0304-3940(96)12943-8. [DOI] [PubMed] [Google Scholar]
- Butson C, McIntyre C. Role of electrode design on the volume of tissue activation during deep brain stimulation. J Neural Engineering. 2006;3:1–8. doi: 10.1088/1741-2560/3/1/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clinical Neurophysiology. 2006;117:447–454. doi: 10.1016/j.clinph.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, McIntyre CC. Tissue and electrode capacitance reduce neural activation volumes during deep brain stimulation. Clinical Neurophysiology. 2005;116:2490–2500. doi: 10.1016/j.clinph.2005.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell B, Woo M. Further studies on asynchronous firing and block of peripheral nerve conduction. Bull Los Angel Neuro Soc. 1966;31:63–71. [PubMed] [Google Scholar]
- Casey K, Blick M. Observations on anodal polarization of cutaneous nerve. Brain Research. 1969;13:155–167. doi: 10.1016/0006-8993(69)90150-4. [DOI] [PubMed] [Google Scholar]
- De Col R, Messlinger K, Carr R. Conduction velocity is regulated by sodium channel inactivation in unmeylinated axons innervating the rat cranial meninges. J Physiol. 2008;586:1089–1103. doi: 10.1113/jphysiol.2007.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostrovsky J, Lozano A. Mechanisms of deep brain stimulation. Movement Disorders. 2002;17:S63–S68. doi: 10.1002/mds.10143. [DOI] [PubMed] [Google Scholar]
- Feng Z, Durand DM. Low-Calcium Epileptiform Activity in the Hippocampus In Vivo. J Neurophysiol. 2003;90:2253–2260. doi: 10.1152/jn.00241.2003. [DOI] [PubMed] [Google Scholar]
- Garcia L, Audin J, D'Alessandro G, Bioulac B, Hammond C. Dual Effect of High-Frequency Stimulation on Subthalamic Neuron Activity. J Neurosci. 2003;23:8743–8751. doi: 10.1523/JNEUROSCI.23-25-08743.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thopmson K, Henderson J, Deisseroth K. Optical Deconstruction of Parkinsonian Neural Circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill W, Mortimer J. Stimulus waveforms for selective neural stimulation. IEEE Engineering in Medicine and Biology. 1995;14:375–385. [Google Scholar]
- Gutnick MJ, Heinemann U, Lux HD. Stimulus induced and seizure related changes in extracellular potassium concentration in cat thalamus (VPL) Electroencephalography and Clinical Neurophysiology. 1979;47:329–344. doi: 10.1016/0013-4694(79)90284-0. [DOI] [PubMed] [Google Scholar]
- Hablitz JJ, Lundervold A. Hippocampal Excitability and Changes in Extracellular Potassium. Exp Neurol. 1981;71:410–420. doi: 10.1016/0014-4886(81)90099-6. [DOI] [PubMed] [Google Scholar]
- Hardesty DE, Sackeim HA. Deep Brain Stimulation in Movement and Psychiatric Disorders. Biological Psychiatry. 2007;I61:831–835. doi: 10.1016/j.biopsych.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the Subthalamic Nucleus Changes the Firing Pattern of Pallidal Neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Lux HD. Ceiling of Stimulus Induced Rises in Extracellular Potassium Concentration in the Cerebral Cortex of Cat. Brain Res. 1977;120:231–249. doi: 10.1016/0006-8993(77)90903-9. [DOI] [PubMed] [Google Scholar]
- Houeto J, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, Welter M, Navarro S, Agid Y. Tourette's syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry. 2005;76:992–995. doi: 10.1136/jnnp.2004.043273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung SW, Hamani C, Lozano AM, Poon Y-YW, Piboolnurak P, Miyasaki JM, Lang AE, Dostrovsky JO, Hutchison WD, Moro E. Long-term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology. 2007;68:457–459. doi: 10.1212/01.wnl.0000252932.71306.89. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Anderson TR, Hu B, Kiss ZH. Cellular mechanisms preventing sustained activation of cortex during subcortical high frequency stimulation. J Neurophysiol. 2006;96:613–621. doi: 10.1152/jn.00105.2006. [DOI] [PubMed] [Google Scholar]
- Jensen A, Durand D. Fiber tract stimulation modulates axonal transmission; 37th annual meeting of the Society for Neuroscience; San Diego, CA. 2007a. [Google Scholar]
- Jensen A, Durand D. Suppression of axonal conduction by sinusoidal stimulation in rat hippocampus in vitro. J Neural Engineering. 2007b;4:1–16. doi: 10.1088/1741-2560/4/2/001. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, Lozano AM, Mayberg HS. Anatomical Connectivity of the Subgenual Cingulate Region Targeted with Deep Brain Stimulation for Treatment-Resistant Depression. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm167. EPublication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, McIntyre C. Quantifying the Neural Elements Activated and Inhibited by Globus Pallidus Deep Brain Stimulation. J Neurophysiol. 2008;100:2549–2563. doi: 10.1152/jn.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Pavlides C. Long-Term Potentiation/Depotentiation Are Accompanied by Complex Changes in Spontaneous Unit Activity in the Hippocampus. J Neurophysiol. 2000;84:1894–1906. doi: 10.1152/jn.2000.84.4.1894. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, Peloquin P, Leung LS. Apical and Basal Orthodromic Population Spikes in Hippocampal CA1 In Vivo Show Different Origins and Patterns of Propagation. J Neurophysiol. 2001;86:2435–2444. doi: 10.1152/jn.2001.86.5.2435. [DOI] [PubMed] [Google Scholar]
- Kocsis J, Malenka R, Waxman S. Effects of extracellular potassium concentration on the excitability of the parallel fibres of the rat cerebellum. J Physiol (Lond) 1983;334:225–244. doi: 10.1113/jphysiol.1983.sp014491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncel A, Grill W. Selection of stimulus parameters for deep brain stimulation. Clinical Neurophysiology. 2004;115:2431–2441. doi: 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Lee K, Hitti F, Shalinsky M, Kim U, Letter J, Roberts D. Abolition of spindle oscillations and 3-Hz absence seizure-like activity in the thalamus by using high-frequency stimulation: potential mechanism of action. J Neurosurg. 2005;103:538–545. doi: 10.3171/jns.2005.103.3.0538. [DOI] [PubMed] [Google Scholar]
- Leung L. Potentials evoked by alvear tract in hippocampal CA1 region of rats. I. Topographical projection, component analysis, and correlation with unit activities. J Neurophysiol. 1979a;42:1557–1570. doi: 10.1152/jn.1979.42.6.1557. [DOI] [PubMed] [Google Scholar]
- Leung L. Potentials evoked by alvear tract in hippocampal CA1 region of rats. II. Spatial field analysis. J Neurophysiol. 1979b;42:1571–1589. doi: 10.1152/jn.1979.42.6.1571. [DOI] [PubMed] [Google Scholar]
- Li S, Arbuthnott G, Jutras M, Goldberg J, Jaeger D. Resonant Antidromic Cortical Circuit Activation as a Consequence of High-Frequency Subthalamic Deep-Brain Stimulation. J Neurophysiol. 2007;98:3525–3537. doi: 10.1152/jn.00808.2007. [DOI] [PubMed] [Google Scholar]
- Lian J, Bikson M, Sciortino C, Stacey WC, Durand DM. Local suppression of epileptiform activity by electrical stimulation in rat hippocampus in vitro. J Physiol (Lond) 2003;547:427–434. doi: 10.1113/jphysiol.2002.033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78:535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- Martin P, Shapiro M. Disparate effects of long-term potentiation on evoked potentials and single CA1 neurons in the hippocampus of anesthetized rats. Hippocampus. 2000;10:207–212. doi: 10.1002/1098-1063(2000)10:3<207::AID-HIPO1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McIntyre C, Grill W, Sherman D, Thakor N. Cellular effects of deep brain stimulation: Model-based analysis of activation and inhibition. J Neurophysiology. 2004a;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- McIntyre C, Savasta M, Goff L, Vitek J. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clinical Neurophysiology. 2004b;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Jiang X, Mennerick S. Action potential fidelity during normal and epileptiform activity in paired soma/axon recordings from rat hippocampus. J Physiol (Lond) 2005;566:425–441. doi: 10.1113/jphysiol.2005.089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Selective Effects of Potassium Elevations on Glutamate Signaling and Action Potential Conduction in Hippocampus. J Neurosci. 2004;24:197–206. doi: 10.1523/JNEUROSCI.4845-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DR, Bikson M, Jefferys JGR. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. Journal of Neuroscience Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Parent M, Butson C, Hahn P, Russo G, Vitek J, McIntyre C. Computational analysis of subthalamic nucleus and lenticular fasiculus activation during therapeutic deep brain stimulation. J Neurophysiol. 2006;96:1569–1580. doi: 10.1152/jn.00305.2006. [DOI] [PubMed] [Google Scholar]
- Mori M, Abegg M, Gahwiler B, Gerber U. A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature. 2004;431:453–456. doi: 10.1038/nature02854. [DOI] [PubMed] [Google Scholar]
- O'Suilleabhain PE, Frawley W, Giller C, Dewey RB., Jr Tremor response to polarity, voltage, pulsewidth and frequency of thalamic stimulation. Neurology. 2003;60:786–790. doi: 10.1212/01.wnl.0000044156.56643.74. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Mauk MD, Kocsis JD. Activity-evoked increases in extracellular potassium modulate presynaptic excitability in the CA1 region of the hippocampus. J Neurophysiol. 1987;58:404–416. doi: 10.1152/jn.1987.58.2.404. [DOI] [PubMed] [Google Scholar]
- Raastad M, Shepherd G. Single-axon action potentials in the rat hippocampal cortex. J Physiol (Lond) 2003;548:745–752. doi: 10.1113/jphysiol.2002.032706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzone M, Lanotte M, Bergamasco B, Tavella A, Torre E, Faccani G, Melcarne A, Lopiano L. Deep brain stimulation of the subthalamic nucleus in Parkinson's disease: effects of variation in stimulation parameters. J Neurol Neurosurg Psychiatry. 2001;71:215–219. doi: 10.1136/jnnp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller Y, Bankirer Y. Cellular Mechanisms Underlying Antiepileptic Effects of Low- and High-Frequency Electrical Stimulation in Acute Epilepsy in Neocortical Brain Slices In Vitro. J Neurophysiol. 2007;97:1887–1902. doi: 10.1152/jn.00514.2006. [DOI] [PubMed] [Google Scholar]
- Shin D, Samoilova M, Cotic M, Zhang L, Brotchie J, Carlen P. High Frequency Stimulation or Eleveated K+ Depresses Neuronal Activity in the Rat Entopeduncular Nucleus. Neuroscience. 2007;149:68–86. doi: 10.1016/j.neuroscience.2007.06.055. [DOI] [PubMed] [Google Scholar]
- Shin DS-H, Carlen PL. Enhanced Ih depresses rat entopeduncular nucleus neuronal activity from high frequency stimulation or raised K+e 10.1152/jn.01065.2007. J Neurophysiol. 2008 doi: 10.1152/jn.01065.2007. 01065.02007. [DOI] [PubMed] [Google Scholar]
- Soleng A, Baginskas A, Andersen P, Raastad M. Activity-dependent excitability changes in hippocampal CA3 cell Schaffer axons. J Physiol (Lond) 2004;560:491–503. doi: 10.1113/jphysiol.2004.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, LeBeau FEN, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annual Review of Neuroscience. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- Velasco F, Velasco M, Velasco A. Centromedian-thalamic and hippocampal electrical stimulation for the control of intractable epileptic seizures. J Clin Neurophysiol. 2001a;18:495–513. doi: 10.1097/00004691-200111000-00001. [DOI] [PubMed] [Google Scholar]
- Velasco F, Velasco M, Velasco A, Menez D, Rocha L. Electrical stimulation for epilepsy: stimulation of hippocampal foci. Stereotact Funct Neurosurg. 2001b;77:223–227. doi: 10.1159/000064610. [DOI] [PubMed] [Google Scholar]
- Vitek J. Mechanisms of deep brain stimulation: Excitation or inhibition. Movement Disorders. 2002;17:S69–S79. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Bracci E, Jefferys JGR. Layer-specific pyramidal cell oscillations evoked by tetanic stimulation in the rat hippocampal area CA1 in vitro and in vivo. J Physiol (Lond) 2005;562:149–164. doi: 10.1113/jphysiol.2004.075390. [DOI] [PMC free article] [PubMed] [Google Scholar]