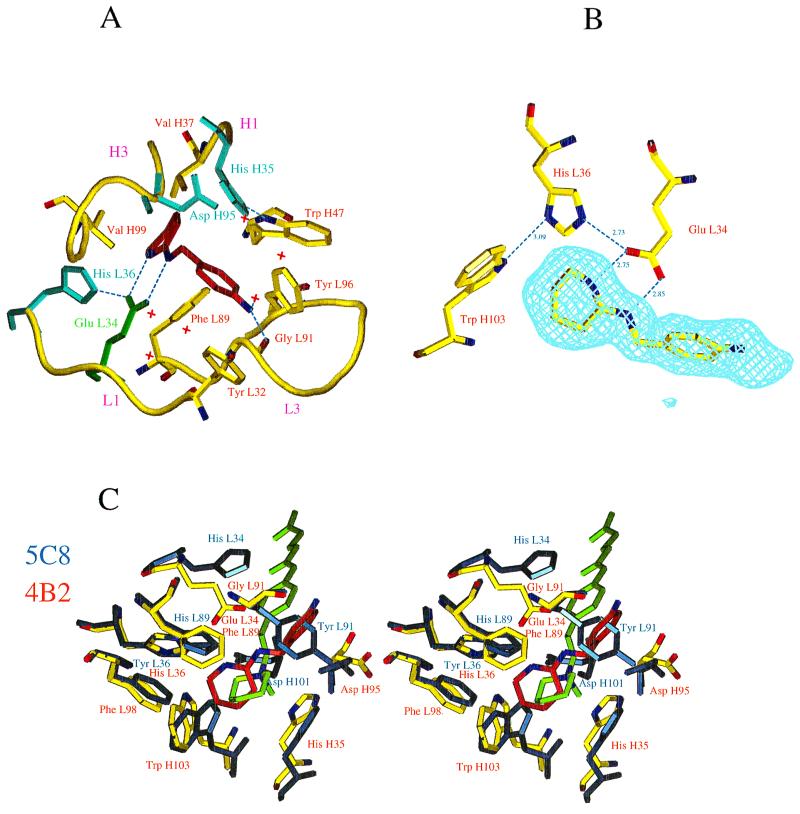
Figure 2.
(A) Schematic view of the active site of the 4B2-1b complex. The ligand is in red; water molecules are indicated as red crosses; Glu L34 is indicated in green; the other polar residues are represented in blue. The Cαs of residues L32–L36, L89–L97, H35–H37, and H94–H99 in hypervariable loops L1, L3, H1, and H3 are shown in yellow. The aromatic residues (Trp H47, Phe L89, Tyr L96, Tyr L32, Trp H103, and Phe L98) that have hydrophobic interactions with the hapten have been represented, except Phe L98 and Trp H103 for reasons of clarity. Nitrogen Nδ1 of His H35 is involved in a conserved H bond with Nɛ1 of Trp H47. His H35 is therefore neutral but cannot play the role of a general base, because its protonated nitrogen Nɛ2 points toward the inside of the cavity. (B) Hydrogen bonding network established with catalytic residue Glu L34. Hydrogen bonds are shown as dashed lines. A Fobs-Fcalc electron density map calculated without the amidinium ligand is superimposed on the structure. The map is contoured at the level of two standard deviations. One of the oxygens of Glu L34 is hydrogen bonded to the protonated nitrogen Nɛ2 of His L36. This tautomeric form of His L36 is stabilized by an additional hydrogen bond between its Nδ1 and the NHɛ1 of Trp H103. Residue His L36 is therefore neutral but cannot play the role of a general base, because its unprotonated nitrogen Nɛ1 points away from the substrate. (C) Comparison of the environment of the charge of the hapten in antibodies 5C8 (in blue) and 4B2 (in yellow). 5C8 catalyzes the disfavored cyclization of an epoxyalcohol (12). The α-carbons of residues of the combining site (L32–L38, L43–L50, L86–L93, L96, L98, H32–H39, H91–H95, and H102–H104) of 5C8 have been superimposed on those of 4B2 (the rms deviation is 0.645 Å). The bottom of the active sites is formed by identical residues in both antibodies (Phe L98, Trp H47, His H35, Trp H103, and Val H37). The distance between the nitrogen of 5b of 5C8 (light green) and the carbon between the two nitrogens of 1b of 4B2 (red) is 1.05 Å. In addition to the ionic interaction with glutamate L34, the hapten amidinium charge of 4B2 is stabilized through a cation–π interaction with Phe L89, which is also involved in a stacking interaction with the amidine cycle. Residues of 5C8 involved in cation–π interaction with the quaternary amine of 5b are (distance to the nitrogen) Tyr L91 (4.97 Å), Trp H103 (5.59 Å), His H35 (4.55 Å), His L89 (4.96 Å), and Tyr L36 (5.39 Å). In addition, in 5C8, Asp H101 (for which there is no equivalent in 4B2 because of the short H3 loop of this antibody) and Asp H95, which are, respectively, 4 Å and 3.7 Å away from the positive charge, provide a second sphere polar environment to the quaternary amine (12). Trp H47, Val H37, and Tyr L96 are not represented for reasons of clarity.