Table 3.
NHC–Zn-catalyzed Enantioselective Allylic Alkylation Reactions of Trisubstituted Olefins with Dialkylzinc Reagentsa
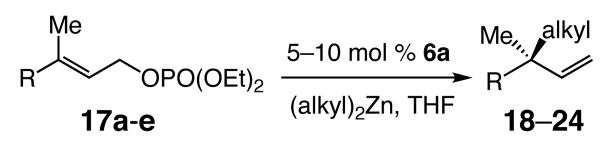 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| entry | substrate (R) | (alkyl)2Zn | product | mol (%) 6a | temp (°C) | time (h) | conv (%);b yield (%)c | SN2′:SN2b | erd |
| 1 | 17a (Ph) | Et2Zn | 18 | 10 | −30 | 48 | 95; 91 | >98:<2 | 95.5:4.5 |
| 2 | 17a (Ph) | n-Bu2Zn | 19 | 10 | −30 | 72 | 72; 71 | >98:<2 | 95.5:4.5 |
| 3 | 17a (Ph) | i-Pr2Zn | 20 | 5 | 0 | 24 | 64; 52 | >98:<2 | 95.5:4.5 |
| 4 | 17b (p-CF3C6H4) | Et2Zn | 21 | 5 | −30 | 48 | >98; 68 | >98:<2 | 94.5:5.5 |
| 5 | 17c (p-NO2C6H4) | Et2Zn | 22 | 10 | −30 | 48 | >98; 82 | >98:<2 | 94.5:5.5 |
| 6 | 17d (Cy) | Et2Zn | 23 | 5 | −15 | 48 | 72; 58 | >98:<2 | 97.5:2.5 |
| 7 | 17e (CO2t-Bu) | n-Bu2Zn | 24 | 5 | −15 | 48 | >98; 85 | >98:<2 | 94:6 |
Reactions were performed under N2 atmosphere.
Conversion and site selectivity were determined by analysis of 400 MHz 1H NMR spectra of product mixtures prior to purification.
Yields of purified products.
Enantiomer ratios were determined by GLC analysis; see the Supporting Information for details.
