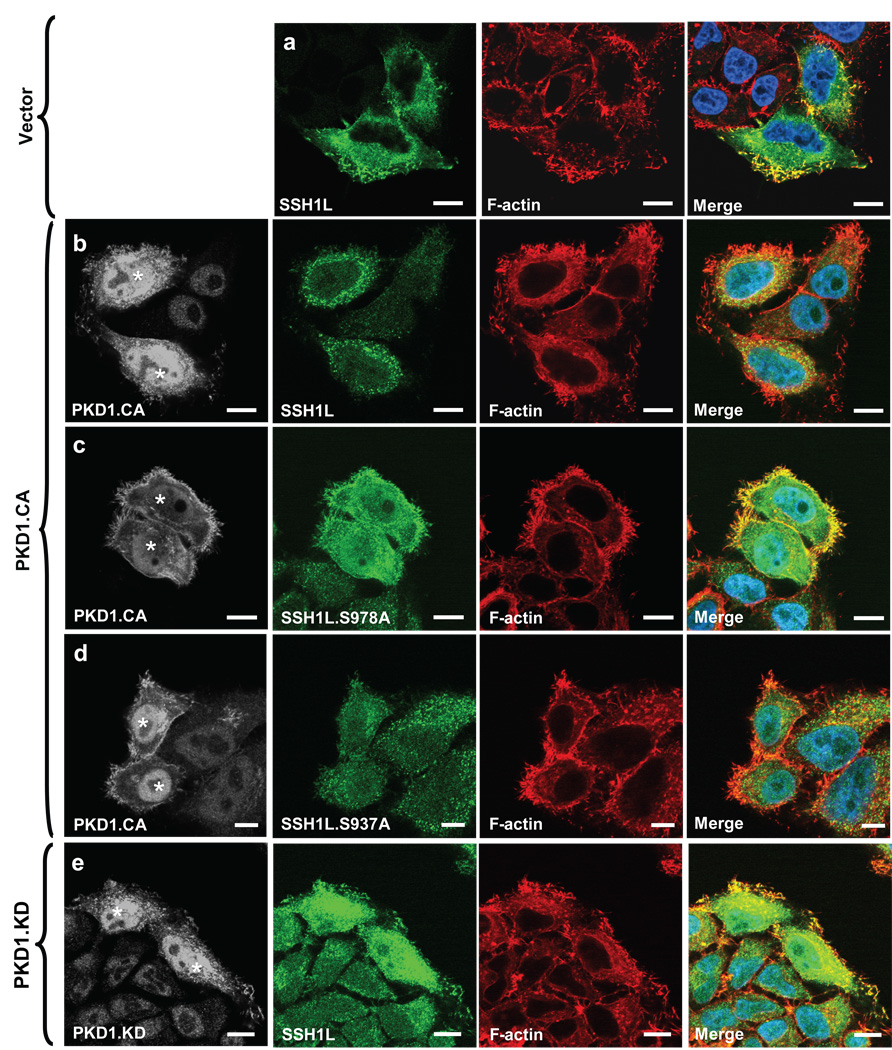
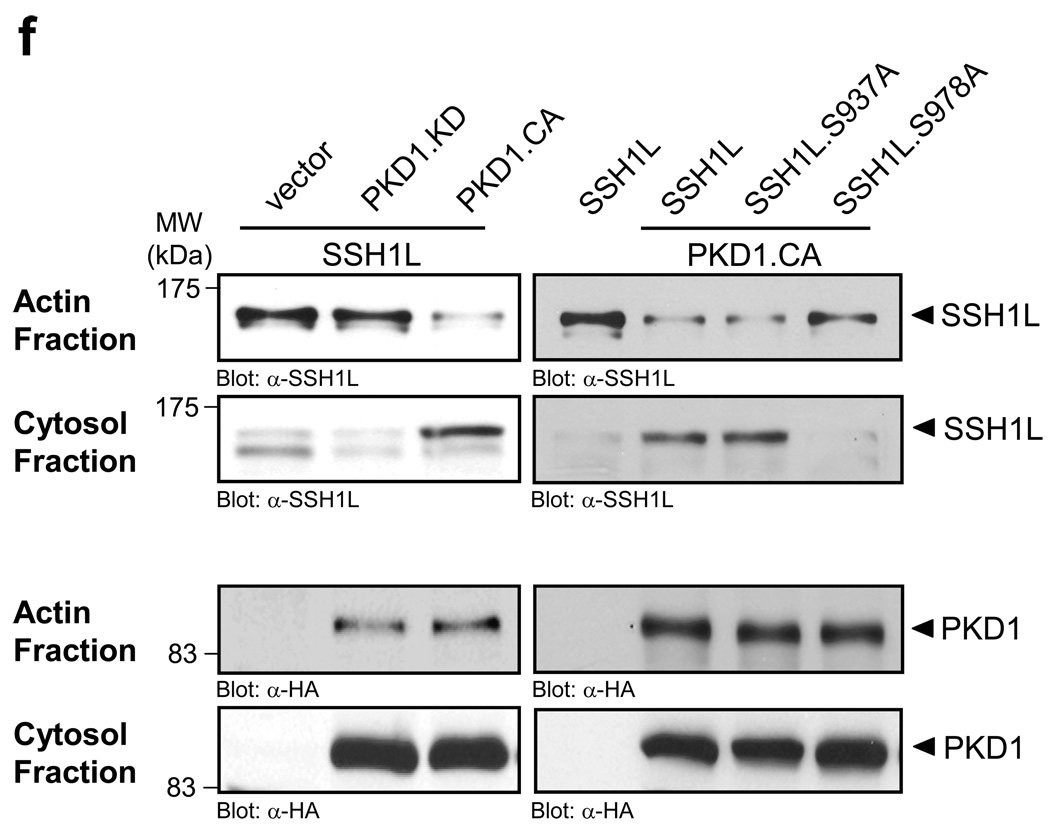
Figure 4. PKD1-mediated phosphorylation regulates SSH1L localization.
a–e, HeLa cells were co-transfected with vector control (a), constitutively-active PKD1 (PKD1.CA) (b–d) or kinase-dead PKD1 (PKD1.KD) (e) and Myc-tagged SSH1L, SSH1L.S937A orSSH1L.S978A (as indicated). Samples were subjected to indirect immunofluorescence analysis. SSH1L was detected using α-myc and Alexa Fluor 568 secondary antibodies. F-actin was stained with Alexa Fluor 633-Phalloidin and nuclei were stained with DAPI. PKD1 expression was detected using a HA-specific antibody and α-rabbit-Alexa Fluor 488 as a secondary antibody. The nuclear staining in the PKD1 samples is non-specific background. Samples were analyzed using a Zeiss LSM 510META confocal microscope with a Plan-Apochromat 63×/1.4 Dic oil immersion objective in Multi-Track-configuration. For visualization purposes, PKD1 images are presented in grayscale, SSH1L in green and Phalloidin in red. Double transfected cells (PKD1 and SSH1L) are marked with asterisks. Images shown depict single confocal sections. The scale bar represents 10 µm. f, HeLa cells were co-transfected with vector control, constitutively-active PKD1 (PKD1.CA) or kinase-dead PKD1 (PKD1.KD) and Myc-tagged SSH1L, SSH1L.S937A or SSH1L.S978A as indicated. Actin fraction or cytosolic fraction of cells were prepared and analyzed for SSH1L (α-SSH1L). Control blots for PKD1 expression were performed using α-HA antibodies. Uncropped images of Fig. 4f are shown in supplementary information Fig. S6. Control figures for 4b, 4c and 4d are shown in Supplementary information S3A.