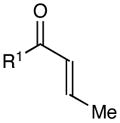
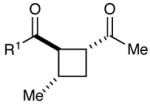
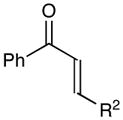
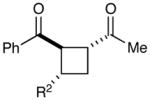
Table 1.
Effect of aryl enone structure in photocatalytic crossed cycloadditions with methyl vinyl ketone 2a
| entry | enone | cycloadduct | yieldb | d.r.b |
|---|---|---|---|---|
 |
 |
|||
| 1 | R1 = Ph | 84% | >10:1 | |
| 2 | R1 = 4-ClPh | 82% | >10:1 | |
| 3 | R1 = 4-MeOPh | 53% | >10:1 | |
| 4 | R1 = 2-furyl | 74% | >10:1 | |
| 5 | R1 = Et | 0% | -- | |
 |
 |
|||
| 6 | R2 = Et | 70% | 6:1 | |
| 7c | R2 = i-Pr | 64% | >10:1 | |
| 8d | R2 = t-Bu | 8% | >10:1 | |
| 9 | R2 = CH2OBn | 61% | >10:1 | |
Unless otherwise noted, reactions conducted using 2.5 equiv 2 and 5 mol% Ru(bipy)3Cl2 in the presence of LiBF4 and i-Pr2NEt in MeCN. Irradiation time using a 23 W compact fluorescent bulb at 30 cm was 4 h.
Isolated yields and diastereomer ratios are the averaged results of two reproducible experiments.
12 h irradiation.
24 h irradiation.
