Fig. 3.
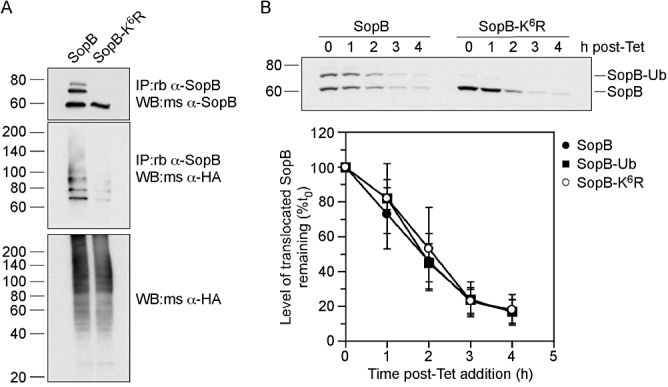
Ubiquitination of SopB does not alter its stability. A. Translocated SopB-K6R is defective for ubiquitination. HeLa cells expressing HA-ubiquitin were infected with ΔsopB-sigE Salmonella expressing plasmid-borne SopB (SopB) and SigE, or SopB with six lysine mutations (SopB-K6R) and SigE. At 2 h p.i., cells were solubilized and post-nuclear supernatants incubated with rabbit polyclonal anti-SopB antibodies followed by Protein A Sepharose beads. Bound proteins were eluted, separated by SDS-PAGE and subject to immunoblotting with mouse polyclonal anti-SopB antibodies or mouse monoclonal anti-HA antibodies. Whole cell lysates were also probed with anti-HA antibodies. B. Ubiquitination does not affect the stability of translocated SopB. HeLa cells were infected with ΔsopB-sigE Salmonella harbouring pWSKDE-GSK or pWSKDE-K6R-GSK. At 2 h p.i., 10 μg ml−1 tetracycline was added to the infected monolayers to stop bacterial protein synthesis and whole cell lysates were collected at hourly intervals by adding boiling 1.5× SDS-PAGE sample buffer. The stability of translocated SopB-GSK was monitored by immunoblotting with rabbit polyclonal antibodies against phospho-GSK. Graph shows densitometric analysis from three separate experiments (mean ± SD).
