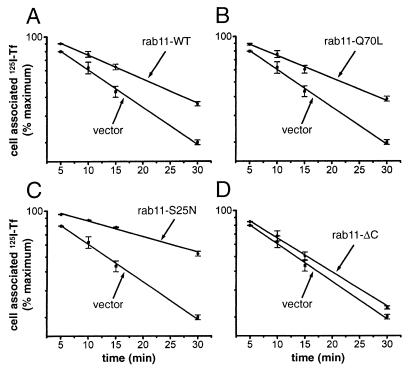
Figure 2.
The constitutively active (Q70L) and wild-type rab11, as well as the dominant negative (S25N) form of this protein, inhibit the recycling of transferrin molecules internalized at 37°C. TRVb cells were cotransfected with pairs of plasmids, one encoding the human transferrin receptor and the other encoding a form of rab11, as indicated in each panel (A, Rab11-wt; B, Rab11-Q70L; C, Rab11-S25N; and D, Rab11-ΔC). The transfected cells were incubated with 125I-transferrin for 1 h at 37°C, to fully load the various intracellular compartments. The cells then were washed, stripped of surface-bound ligand, and incubated at 37°C in medium containing unlabeled transferrin. The extent of release of the internalized marker into the medium was monitored at various times thereafter. The percentage of 125I-transferrin that remains cell-associated in cells expressing each rab11 variant is shown in a semi-log plot as a function of time, together with equivalent data from control cells transfected with the vector lacking a rab11 cDNA insert. Except for rab11-ΔC, the data represent the averages of two separate experiments. The straight lines were obtained by nonlinear curve fitting to the equation y = A* exp (−Kr ⋅ t), where y is the percent of cell-associated 125I-transferrin and Kr the apparent first order recycling rate constant. The rate constants for the different transfected cultures are listed in Table 1.