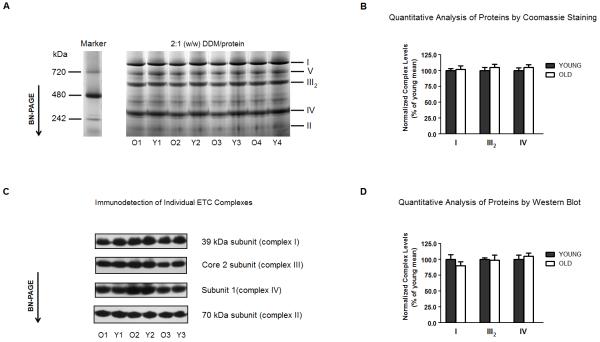
Figure 1.
The levels of individual electron transport complexes of rat heart interfibrillary mitochondria do not change with age. (A) Heart interfibrillar mitochondria were isolated from young (Y: 5 months, n = 4) and old (O: 24 months, n = 4) Fischer 344 rats, and membranes were solubilized with a DDM-to-protein ratio of 2:1 (w/w). NativeMark (Invitrogen, Carsbad, CA, USA) was used as a molecular weight standard for proteins separated by BN-PAGE. (B) Levels of complexes I, III and IV were calculated using the density of Coomassie-stained proteins. In order to control for differences in protein-loading, the density of each complex was normalized to the density of complex II from the corresponding lane. (C) In separate experiments, mitochondrial proteins were separated by BN-PAGE as indicated in (A), and Western blot analysis was used for identification of individual electron transport complexes. Mitochondrial complexes were detected using monoclonal antibodies against the following subunits: 39 kDa of complex I, 70 kDa of complex II, core 2 of complex III and subunit I of complex IV, as described in Materials and Methods. (D) Levels of complexes I, III and IV were calculated using densitometric analysis after identification of proteins by Western blot. In order to control for differences in protein-loading, the density of each complex was normalized to the density of complex II from the corresponding lane. I, II, III2, IV and V denote individual OXPHOS complexes. All results are presented as the mean ± SEM, and plotted as a percentage of the mean from young controls.