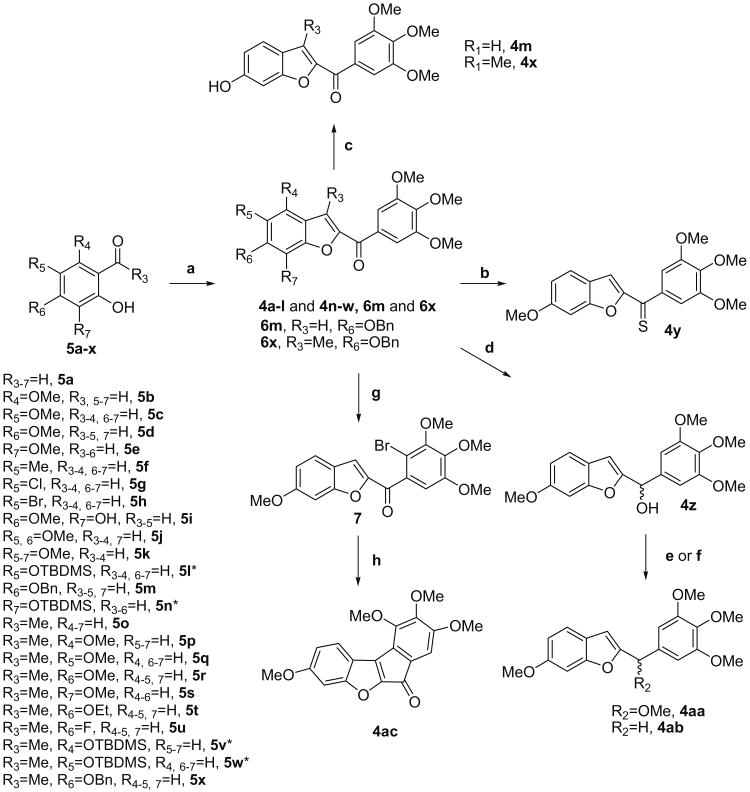
Scheme 1.
Reagents and conditions: (a) (3,4,5-Trimethoxyphenyl)-2-bromo-ethanone, K2CO3, (CH3)2CO, rt; (b) Lawesson's reagent, THF, rt from 4d; (c) HCO2NH4,10% Pd/C, MeOH, rt from 6m and 6x; (d) NaBH4, MeOH, rt from 4d; (e) PTSA, MeOH-THF, rt; (f) Et3SiH, TFA, CH2Cl2, rt; (g) NBS, benzoyl peroxide, MeCN, rt from 4d; (h) Pd(Ph3P)4, KOAc, DMA, 130 °C. For compounds 5l, 5n and 5v–w, condition ‘a’ led to a cyclization with concomitant removal of TBDMS group, to afford 4l, 4n and 4v–w, respectively.