Abstract
We investigated the association between plasma HIV-1 RNA, immune activation, and polyclonal T cell function in viremic subjects whether on or off antiretroviral therapy (ART). The surface expression of activation/functional molecules on T cells and monocytes as well as cytokine secretion and T cell proliferation were assessed in 23 HIV-1− and 79 HIV-1+-infected subjects with different levels of viral suppression and CD4+ T cell count >250 cells/mm3 for >6 months. Viral replication was associated with increased T cell and monocyte activation irrespective of ART. In subjects with a detectable viral load on ART, we found a positive association with anti-CD3/CD28-induced T cell proliferation compared to patients with undetectable viral load (<400 copies/ml). No difference among groups was observed for anti-CD3/CD28-mediated IFN-γ responses. The presence of an unexpected positive association between polyclonal T cell proliferation and viral load in subjects with levels of T cell IFN-γ responses comparable to those of uninfected subjects is of potential relevance to an increase in T cell activation response before the loss of polyclonal cytokine secretion and proliferation observed with disease progression. This finding suggests that T cell hyperresponsiveness may play a role in the pathogenesis of immune comorbidities on ART.
HIV-1 infection is associated with generalized immune dysfunction. Among other effects, increased T cell activation,1–6 impaired costimulatory molecule expression (CD80/CD86),7 loss of CD4+ T cell lymphoproliferative responses,8 and decreased cytokine production9–11 have been associated with progressive loss of CD4 count and rising viral load. Furthermore, peripheral blood mononuclear cells (PBMCs) from patients with a low CD4+ T cell count and high viral load express lower levels of interleukin (IL)-13 and interferon (IFN)-γ following ex vivo stimulation by anti-CD3/anti-CD28 antibodies.12
Effective treatment of HIV-1-infected subjects with antiretroviral therapy (ART) results in viral suppression and immune reconstitution, characterized by an increase of CD4+ T cells, decrease in immune activation,13 and recovery of CD4+ T cell recall responses.14 Detectable HIV antigen-specific responses15,16 have also been demonstrated in subjects with low-level viremia, as well as in ART-treated subjects who exhibit a sustained CD4+ T cell count despite virologic failure. Although progressive loss of T cell function has been correlated with disease progression,17 the relationship of ongoing viral replication with T cell and monocyte activation and function in the absence of advanced HIV infection (CD4+ T cell count <250 cells/mm3), irrespective of the presence of ART, remains largely unknown.
HIV-1+ subjects with different levels of viral suppression and CD4+ T cell count >250 cells/mm3 for >6 months before collection were recruited. Venous peripheral blood (80 ml) was collected from 23 HIV-1− subjects and 79 HIV-1+ subjects with plasma HIV-1 RNA <400 copies/ml (n = 34, 29 on ART), 400–5000 copies/ml (n = 28, 18 on ART), and >5000 copies/ml (n = 17, 9 on ART). Plasma HIV-1 RNA was determined at the time of blood draw by Quest Diagnostic (Horsham). Informed consent was obtained according to the Human Experimentation Guidelines of the U.S. Department of Health and Human Services and of the authors' institutions. The study protocol was approved by the Institutional Review Boards (IRBs) of the Wistar Institute and Philadelphia FIGHT.
Cell surface expression of targeted molecules was measured by same-day whole blood staining. Fluorochrome-conjugated antihuman monoclonal antibodies from the following sources were used: (1) BD PharMingen (San Diego): IgG1 CD3-phycoerythrin (PE), IgG1 CD4-PE, IgG1 CD38-PE, IgG1 CD28-fluorescein isothiocyanate (FITC), IgG2a CD14-FITC, IgG1 CD40-FITC, IgG1 CD86-FITC, isotypes mouse IgG1-PE, mouse IgG1-FITC, mouse IgG2a-FITC, mouse IgG2a-Tri-Color (TC), and mouse IgG2b-allophycocyanin (APC), and (2) Invitrogen/CalTag (Burlingame): IgG1 CD80-PE, IgG1 CD4-FITC, IgG1 CD95-FITC, MHC Class I-FITC, IgG2a CD4-TC, IgG2a CD8-TC, and HLA-DR-APC. The following antibody combinations were used: (1) CD3-PE/CD95-FITC/HLA-DR-APC, (2) CD4-TC/CD28-FITC/CD38-PE/HLA-DR-APC, (3) CD8-TC/CD28-FITC/CD38-PE/HLA-DR-APC, (4) CD80-PE/CD86-FITC/HLA-DR-APC, (5) CD40 FITC/HLA-DR-APC, (6) MHC Class I-FITC/HLA-DR-APC, and (7) CD14-FITC/HLA-DR-APC. Color compensation was performed by using CD4-FITC, CD4-PE, CD4-TC, and HLA-DR-APC. Combinations 1–3 were used for phenotypic characterization of T cells, while combinations 4–7 were used for phenotypic characterization of monocytes. Samples were analyzed on a Becton Dickinson FACScalibur flow cytometer using the CellQuest software package for acquisition and analysis as previously described.18 Live cell gates and detection thresholds were set according to isotype-matched negative controls. A minimum of 104 events was collected for each analysis. Nonoverlapping lymphocyte and monocyte gates (separated to avoid activated lymphocyte crossover) were defined/reconfirmed for each subject based on size and granularity [at forward scatter (FSC)/side scatter (SSC)] and CD3 or CD14/HLA-DR expression, respectively. Results were expressed as percent (%) of positive events and as mean fluorescent intensity (MFI) for CD38, CD80, CD86, CD40, and MHC Class I.
Lymphoproliferative assays (LPA) were initiated on the same day as cell isolation, as previously described.18 PBMCs (250,000 cells/well) were cultured in a six replicate format along with medium alone (background), anti-CD3 (OKT3 IgG2a)/anti-CD28 ascites (CK-248 IgM), or phytohemagglutinin (PHA, 5 μg/ml, Sigma-Aldrich), as previously described.12 Results were expressed as stimulation index (SI = antigen stimulated mean cpm/unstimulated mean cpm). An SI >3 was interpreted as positive.
For cytokine assessment, PBMCs were stimulated with anti-CD3/CD28 on the same day as isolation, and supernatants were collected at day 3, as previously described.12 IFN-γ was measured by in-house RIA, as previously described,12 while IL-13 was measured by ELISA according to the manufacturer's specifications. All cytokine measurements were based on the average of triplicate (RIA) or duplicate (ELISA) samples. Thresholds of detection were 1.6 pg/ml for IFN-γ and 7.813 pg/ml for IL-13.
The data are described as medians and 25th and 75th percentiles for all groups. Each variable distribution was analyzed for normality using the Shapiro-Wilk W test (p > 0.05) and between group comparisons were performed by t-test or the Wilcoxon/Kruskal-Wallis test (rank sums) depending on the data distribution. Correlations between variables were assessed using Spearman or pairwise correlation tests. Descriptive analyses and statistical tests were performed using JMP 4.0 (SAS Institute) and study conclusions were reconfirmed following multiple testing correction.
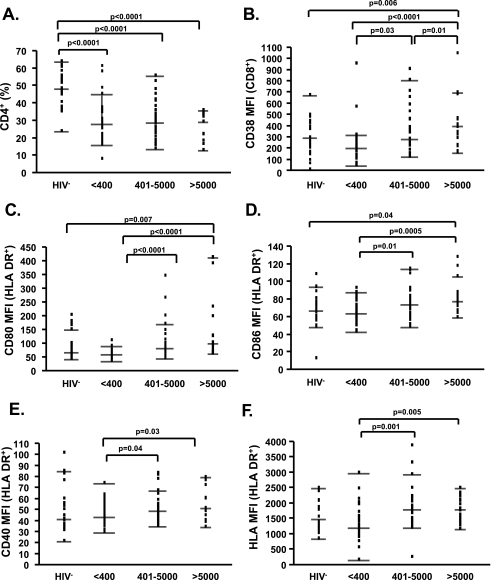
The median age of the subjects was 40 years (25th–75th percentile 23–66); 48% of HIV-1− and 59% of HIV-1+ subjects were male. The median CD4% of all HIV-1− subjects was 48 and of all HIV+ subjects was 28% (Fig. 1A).
FIG. 1.
Increased T cell and monocyte activation as a result of viremia. (A) Percentages of CD4+ T cells are shown in HIV-1− subjects and in HIV-1+ subjects with plasma HIV-1 RNA <400, 400–5000, and >5000 copies/ml irrespective of ART status. (B–F) Mean fluorescent intensity (MFI) of CD38 on CD8+ T cells (B) and of CD80 (C), CD86 (D), CD40 (E), and HLA (F) on HLA-DR+ monocytes is shown in similar groups as in (A). Dots represent individual patients data, while lines indicate the median and 25th and 75th percentiles. Significant p values are shown on the top of each graph.
Similar to what has been described by several groups,19 viral replication was positively associated with T cell activation, as characterized by assessment of the percent of CD3+/CD95+ (p = 0.04, rho = 0.29), CD3+/HLA-DR+ (p = 0.002, rho = 0.43), CD3+/CD95+/HLA-DR+ (p = 0.002, rho = 0.44), CD4+/CD38+ (p = 0.02, rho = 0.33), and CD8+/CD38+ (p = 0.0004, rho = 0.51). These results were further supported by between-group comparisons for CD38 MFI changes (Fig. 1B). Viral replication also resulted in significantly increased levels of CD80, CD86, CD40, and MHC Class I MFI on monocytes (Fig. 1C–F), while no significant difference was observed among groups in the percent of CD80+/HLA-DR+, CD86+/HLA-DR+, CD40+/HLA-DR+, and MHC Class I+/HLA-DR+ cells. Interestingly, the direct association between T cell activation, monocyte cell surface modulation, and HIV-1 replication was present irrespective of ART (data for percent of CD8+/CD38+ T cells shown in Fig. 2C).
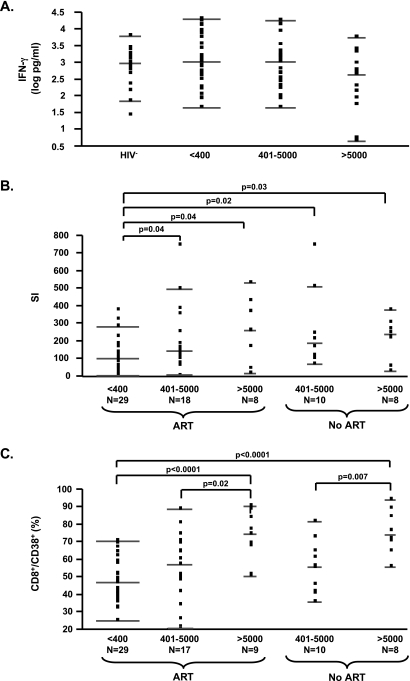
FIG. 2.
Polyclonal T cell proliferation with increasing viral load in subjects with T cell cytokine responses comparable to uninfected subjects. (A) Anti-CD3/CD28-mediated IFN-γ responses (following subtraction of the unstimulated levels, expressed as log pg/ml) in HIV-1− subjects and in HIV-1+ subjects with plasma HIV-1 RNA <400, 400–5000, and >5000 copies/ml irrespective of ART status. (B) Anti-CD3/CD28 T cell responses (expressed as SI) in HIV-1+ subjects with plasma HIV-1 RNA <400, 400–5000, and >5000 copies/ml in the presence or absence of ART. (C) Percentages of CD8+/CD38+ T cells in HIV-1+ subjects in groups as summarized in (B). Dots represent individual patients data, while lines indicate the median and 25th and 75th percentiles. Significant p values are shown on the top of each graph.
Subsequently, we evaluated functional outcomes of T cell activation in PBMCs for the same subjects by analyzing anti-CD3/CD28-dependent cytokine and proliferative responses. IFN-γ and IL-13, measured as cytokines, were previously described to be affected by disease progression when evaluated by anti-CD3/CD28 stimulation, as performed here.12 However, we observed no significant difference in IFN-γ or IL-13 secretion levels between HIV+-infected groups (data for IFN-γ shown in Fig. 2A) even when subjects were segregated based on the presence or absence of ART (not shown) or based on CD4 T cell frequency (above or below a median CD4% of 28). Higher cytokine secretion was observed in HIV-1− subjects (IFN-γ: median 965.4, 25th–75th percentile 401.6–1978.1; IL-13: median 1507.2, 25th–75th percentile 227–2514.5) as compared to HIV-1+ patients with plasma HIV-1 RNA >5000 copies/ml in the absence of ART (IFN-γ: median 441.3, 25th–75th percentile 4.7–1692.7; IL-13: median 139.7, 25th–75th percentile 22.3–304.5) as previously described.12 Significance was reached only with induced IL-13 levels (p = 0.0017). The comparable levels of anti-CD3-/CD28-mediated IFN-γ and IL-13 responses between viremic and uninfected subjects observed in this study suggest that our viremic subject groups with CD4 counts >250 cells/μl were enriched for subjects in early disease.
Surprisingly, following stimulation with anti-CD3/CD28 or PHA, we observed increased T cell proliferation in viremic subjects independent of ART (Fig. 2B). In addition, a positive association between viral load on ART and T cell proliferation upon stimulation with anti-CD3/CD28 (p = 0.0038, rho = 0.38) or PHA (p = 0.0046, rho = 0.37) was observed. Unstimulated proliferation levels were similar, excluding an experimental bias. Interestingly, the increase in proliferation was observed only in viremic subjects with CD4 T cell frequency above the median (CD4% median 28), supporting the interpretation that this effect may be limited to subjects with higher CD4 cell frequencies in conjunction with the presence of T cell cytokine responses. Finally, in order to evaluate whether the increased T cell activation and activation-induced proliferation were associated with documented changes on accessory cells, we tested for an association between monocyte expression of CD80, CD86, and CD40, and the anti-CD3/CD28 T cell proliferation response. No direct association was found between monocyte phenotype frequencies or MFIs measured and levels of T cell proliferation.
Contrary to the negative effects of viral replication on T cell cytokine and proliferative responses in progressive HIV disease, we document, for the first time, a positive association between viral replication and polyclonal T cell proliferative responses in chronically HIV-1-infected patients with CD4 counts >250 cells/μl and sustained IFN-γ secretion responses. Our data suggest that a positive association between viral load and T cell proliferation response is present in subjects with intact polyclonal cytokine secretion responses and precedes the loss of cytokine response by activated T cells and a decline in T cell proliferative responses, which are commonly observed in advanced disease.20 A general upregulation of monocyte coactivation in viremic subjects in conjunction with increased polyclonal T cell proliferation function, as observed here, would be predicted to be associated with higher activation of antigen-specific responses in the presence of viremia. However, it remains to be determined whether an increased polyclonal T cell proliferation response is present with increasing viremia in subjects with intact IFN-γ secretion responses during early non-ART-treated disease and before the decrease of CD4 T cells,21 as predicted by our data.
Of interest, recent reports confirm an exacerbation of autoimmune diseases in the presence of ART.22,23 This suggests that viral replication in the presence of recovered T cell functionality on ART may exacerbate autoreactive T cells, as observed here with polyclonal stimulation. It should be noted that based on the polyclonal stimulation method used, we cannot rule out that the increase in proliferation observed may also include amplification of HIV-specific memory responses aided by residual HIV-1 antigen retained in vitro from viremic subjects.
In conclusion, our findings document an unexpected positive association between polyclonal T cell proliferation responses and viral replication in subjects with T cell cytokine response levels comparable to those of uninfected subjects. Our data also indicate that despite the beneficial effect of ART in achieving immune reconstitution, incomplete viral suppression may promote T cell hyperresponsiveness as a result of accessory and effector cell interactions. This is of relevance to immunotherapy or autoimmune comorbidities on ART.
Acknowledgments
We would like to thank all the subjects who participated in the study, D. Davis for study assistance, clinical providers, and the Board and Staff of Philadelphia FIGHT. No commercial affiliations were present. This work was supported by a grant from the National Institutes of Health AI051986, AI051225, AI047760, the Philadelphia Foundation, Mrs. M. Stengel Miller's support of the HIV-1 Partnership Program for Basic Research, and funds from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health.
References
- 1.Resino S. Navarro J. Bellon JM. Gurbindo D. Leon JA. Munoz-Fernandez MA. Naive and memory CD4+ T cells and T cell activation markers in HIV-1 infected children on HAART. Clin Exp Immunol. 2001;125:266–273. doi: 10.1046/j.1365-2249.2001.01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plaeger-Marshall S. Isacescu V. O'Rourke S. Bertolli J. Bryson YJ. Stiehm ER. T cell activation in pediatric AIDS pathogenesis: Three-color immunophenotyping. Clin Immunol Immunopathol. 1994;71:19–26. doi: 10.1006/clin.1994.1046. [DOI] [PubMed] [Google Scholar]
- 3.Kestens L. Vanham G. Vereecken C, et al. Selective increase of activation antigens HLA-DR and CD38 on CD4+ CD45RO+ T lymphocytes during HIV-1 infection. Clin Exp Immunol. 1994;95:436–441. doi: 10.1111/j.1365-2249.1994.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herbein G. Mahlknecht U. Batliwalla F, et al. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 5.Gehri R. Hahn S. Rothen M. Steuerwald M. Nuesch R. Erb P. The Fas receptor in HIV infection: Expression on peripheral blood lymphocytes and role in the depletion of T cells. AIDS. 1996;10:9–16. [PubMed] [Google Scholar]
- 6.de Martino M. Rossi ME. Azzari C. Gelli MG. Galli L. Vierucci A. Different meaning of CD38 molecule expression on CD4+ and CD8+ cells of children perinatally infected with human immunodeficiency virus type 1 infection surviving longer than five years. Pediatr Res. 1998;43:752–758. doi: 10.1203/00006450-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Dudhane A. Conti B. Orlikowsky T, et al. Monocytes in HIV type 1-infected individuals lose expression of costimulatory B7 molecules and acquire cytotoxic activity. AIDS Res Hum Retroviruses. 1996;12:885–892. doi: 10.1089/aid.1996.12.885. [DOI] [PubMed] [Google Scholar]
- 8.Younes SA. Yassine-Diab B. Dumont AR, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreuzer KA. Dayer JM. Rockstroh JK. Sauerbruch T. Spengler U. The IL-1 system in HIV infection: Peripheral concentrations of IL-1beta, IL-1 receptor antagonist and soluble IL-1 receptor type II. Clin Exp Immunol. 1997;109:54–58. doi: 10.1046/j.1365-2249.1997.4181315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chehimi J. Starr SE. Frank I, et al. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badley AD. Dockrell D. Simpson M, et al. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med. 1997;185:55–64. doi: 10.1084/jem.185.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailer RT. Holloway A. Sun J, et al. IL-13 and IFN-gamma secretion by activated T cells in HIV-1 infection associated with viral suppression and a lack of disease progression. J Immunol. 1999;162:7534–7542. [PubMed] [Google Scholar]
- 13.Autran B. Carcelain G. Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 14.Rinaldo CR., Jr Liebmann JM. Huang XL, et al. Prolonged suppression of human immunodeficiency virus type 1 (HIV-1) viremia in persons with advanced disease results in enhancement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J Infect Dis. 1999;179:329–336. doi: 10.1086/314599. [DOI] [PubMed] [Google Scholar]
- 15.D'Ettorre G. Forcina G. Andreotti M, et al. Discordant response to antiretroviral therapy: HIV isolation, genotypic mutations, T-cell proliferation and cytokine production. AIDS. 2002;16:1877–1885. doi: 10.1097/00002030-200209270-00004. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson AC. Younger SR. Martin JN, et al. Immunologic and virologic evolution during periods of intermittent and persistent low-level viremia. AIDS. 2004;18:981–989. doi: 10.1097/00002030-200404300-00005. [DOI] [PubMed] [Google Scholar]
- 17.Clerici M. Stocks NI. Zajac RA, et al. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papasavvas E. Ortiz GM. Gross R, et al. Enhancement of human immunodeficiency virus type 1-specific CD4 and CD8 T cell responses in chronically infected persons after temporary treatment interruption. J Infect Dis. 2000;182:766–775. doi: 10.1086/315748. [DOI] [PubMed] [Google Scholar]
- 19.Kestens L. Vanham G. Gigase P, et al. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6:793–797. doi: 10.1097/00002030-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Novak RM. Koirala J. Sirdar ML, et al. Lymphoproliferative responses to mitogens and prepared antigens of M. avium complex in patients with HIV infection. J Clin Immunol. 2000;20:62–67. doi: 10.1023/a:1006646711977. [DOI] [PubMed] [Google Scholar]
- 21.Pitcher CJ. Quittner C. Peterson DM, et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 22.Chen F. Day SL. Metcalfe RA, et al. Characteristics of autoimmune thyroid disease occurring as a late complication of immune reconstitution in patients with advanced human immunodeficiency virus (HIV) disease. Medicine (Baltimore) 2005;84:98–106. doi: 10.1097/01.md.0000159082.45703.90. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese LH. Kirchner E. Shrestha R. Rheumatic complications of human immunodeficiency virus infection in the era of highly active antiretroviral therapy: Emergence of a new syndrome of immune reconstitution and changing patterns of disease. Semin Arthritis Rheum. 2005;35:166–174. doi: 10.1016/j.semarthrit.2005.03.007. [DOI] [PubMed] [Google Scholar]