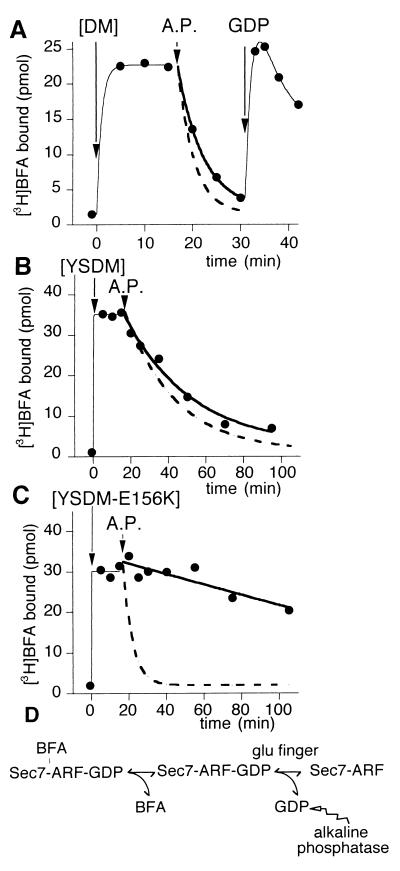
Figure 3.
BFA does not bind to the nucleotide-free complex between [Δ17]ARF1 and Sec7d. The sample (200 μl) contained initially 10 μM [Δ17]ARF1–GDP and 25 μM [3H]BFA. The binding of 25 μM [3H]BFA was initiated at zero time by the addition of Arno-Sec7 (5 μM) carrying either the DM mutations (A), the YSDM mutations (B), or the YSDM + E156K mutations (C). At t = 17 min, alkaline phosphatase (200 units/ml) was added to the sample. In A, 2 mM GDP was further added at t = 31 min. Each experimental point corresponded to the bound radioactivity in a 15-μl aliquot. The continuous thick lines show an exponential fit of the data points after the addition of alkaline phosphatase. The dashed lines report the time course of [3H]BFA dissociation as measured by exchange with unlabeled BFA and measured in Fig. 2. Note the good correlation between the two fits (A and B) except for the complex with [YSDM-E156K]Arno-Sec7 (C). These experiments suggest that once BFA has bound to a Sec7d–ARF1–GDP complex, the release of GDP and its hydrolysis by alkaline phosphatase requires first the dissociation of BFA from the complex and then the destabilization of GDP by the glutamic finger of Sec7d (D).