Figure 3.
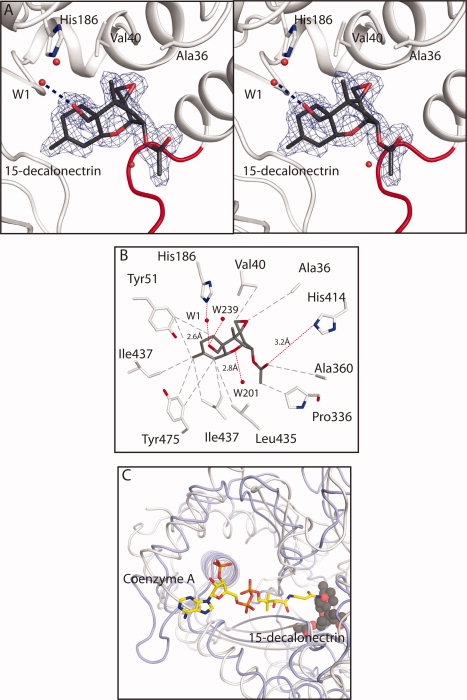
Details of the interaction of trichothecene intermediate 15-decalonectrin with TRI3. (A) Stereoview of the electron density corresponding to 15-decalonectrin (dark gray) bound to TRI3 (white). The map, contoured at 3σ was calculated from coefficients of the form Fo − Fc where the ligand was omitted from the phase calculation and refinement. Residues that interact with the epoxide moiety are depicted as sticks. Loop residues Lys424-Pro434, proposed to allow active site access are colored red. (B) Schematic representation of the interactions made by TRI3 with 15-decalonectrin. Polar and apolar interactions and distances in Å are indicated as red and gray dashes, respectively. Distance between the catalytic His186 and C15 Oxygen is not ideal for hydrogen bonding. (C) Coenzyme A binding site model. The proposed CoA binding site of TRI3 based on a structural superposition of the structure of FgTRI101 complexed with CoA and DON onto the structure of the TRI3·15-decalonectin complex is shown. TRI3 is colored white, FgTRI101 is colored light blue, CoA from the FgTRI101 structure is shown in stick representation, and 15-decalonectrin is depicted with spheres.
