Figure 4.
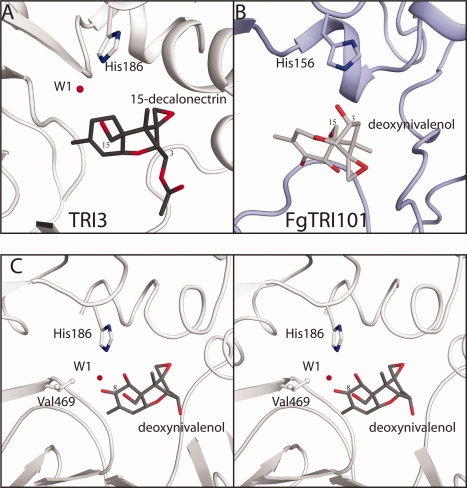
Comparison of trichothecene binding orientations in the structurally homologous TRI3 and FgTRI101. (A) TRI3 complexed with 15-decalonectrin. The 15-decalonectrin epoxide moiety is oriented into a hydrophobic depression formed by residues of the N-terminal domain, this requires a substantial reorientation of the substrate as compared to the TRI101 structures. In this structure the catalytic His186 does not form a hydrogen bond with the C15 hydroxyl moiety, but does form a hydrogen bond with a trapped water molecule. (B) FgTRI101 complexed with CoA and DON (3B2S). The DON substrate's epoxide moiety is oriented toward the C-terminal domain and the catalytic His156 is within ideal hydrogen bonding distance. (C) Proposed model of TRI3 complexed with DON. DON has been superimposed onto the core trichothecene ring structure of the TRI3·15-decalonectrin complex. This binding position of DON in the active site would position the C8 ketone moiety in close proximity to the Val469 side chain, thus creating a poor interaction that accounts for the lack of catalytic activity observed with DON as a substrate.
