Figure 7.
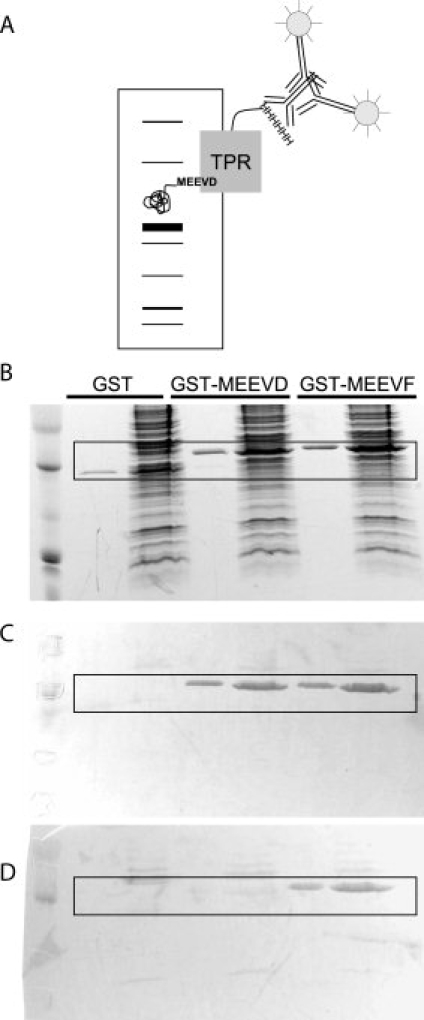
Western Blots using TPRs as Primary Antibodies. (A) Schematic illustrating Western blot strategy. Cellular extract containing a tagged target protein is separated by SDS-PAGE and then transferred to a membrane. The tagged protein is probed with His-TPRs, followed by anti-His antibodies and alkaline-phosphatase conjugated secondary antibodies. (B) A duplicate SDS-PAGE gel used for Western blotting is stained for total protein with Coomassie Brilliant Blue. 1.75 μg of purified tagged protein was diluted in water (lanes 2, 4, and 6) or cellular extract (lanes 3, 5, and 7). (C) Blot probed with His-TPR2A. Note that both the MEEVD and MEEVF tagged proteins are stained. (D) Blot probed with His-T-Mod(MMY). Note that only the MEEVF tagged protein is stained. The boxed areas in B–D highlight the presence or absence of GST-peptide staining.
