Figure 9.
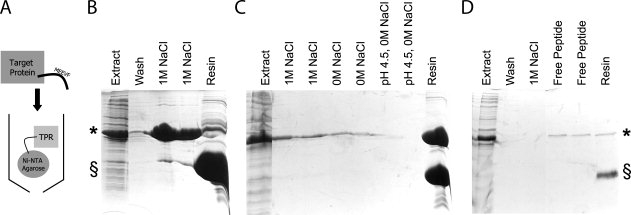
Affinity Purification of Tagged Proteins using TPRs. (A) Schematic illustrating affinity purification strategy. His-TPRs are immobilized on Ni-NTA resin and extracts containing MEEVF tagged target proteins are passed over the resin and purified. (B) Affinity purification of MEEVD tagged protein (*) using TPR2A (§), as illustrated by SDS-PAGE. Soluble lysate containing MEEVD tagged target protein (lane 1) is passed over the resin to bind. Following washes (with 50 mM Tris pH 8.0, 150 mM NaCl, 5 mM B-mercaptoethanol) (lane 2), the MEEVD tagged protein is eluted with wash buffer supplemented to 1M NaCl (lanes 3 and 4). Minimal target protein remains bound to the TPR (lane 5). (C) Affinity purification of MEEVF tagged protein (*) using T-Mod(MMY) (§). The same procedures were followed, except here, the interaction predominantly withstands 1M NaCl (lanes 2–3), 0M NaCl (lanes 4–5), and low pH conditions (lanes 6–7). Almost all of the tagged protein remains bound to the TPR (lane 8). The purification of MEEVF tagged protein using T-Mod(MMY) was repeated (D) and again the interaction withstands 1M NaCl (lane 3) before elution with 100 μg/mL free MEEVF peptide (lanes 4–5). Note that these bands are weaker because of the lower total amount of MEEVF tagged protein, but that far less MEEVF tagged protein remains bound to the TPR, indicating better elution (lane 6).
