Figure 3.
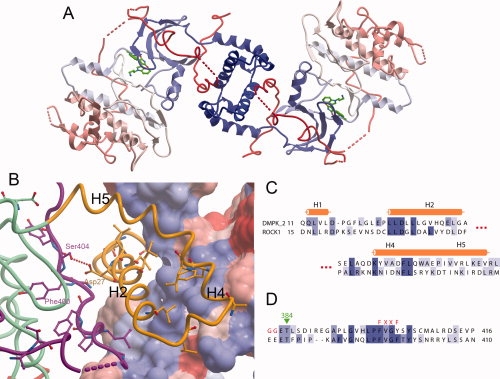
DMPK dimer. (A) The DMPK biological dimer, with each molecule colored blue to red from N- to C-terminus. (B) The dimerisation interface of DMPK. The surface is shown for one DMPK monomer, colored according to hydrophobicity, with blue more hydrophobic and red less hydrophobic. The other DMPK monomer is shown as a ribbon, with residues that are conserved between ROCK1 and DMPK illustrated. The DMPK ribbon is colored as in Figure 1. (C) Partial sequence alignment of the N-terminal regions of DMPK and ROCK1 involved in the dimer interface. (D) Partial sequence alignment of the C-terminal regions of DMPK and ROCK1 involved in the dimer interface.
