Figure 4.
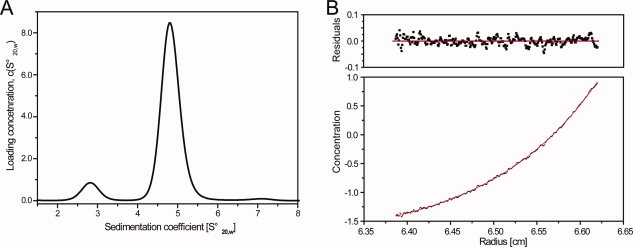
Self association of DMPK studied by analytical ultracentrifugation. (A) Sedimentation velocity experiment, the concentration of DMPK was 25 μM. At that concentration the protein was mainly populated as a dimer as indicated by the peak at 4.8 Swedberg units. The molecular weights determined from the velocity data were ∼48 and ∼100 kDa, respectively, consistent with the expected molecular weight of a DMPK monomer and dimer. (B) Sedimentation equilibrium experiment of DMPK. The upper panel shows residuals to a nonlinear least-squares fit to a monomer–dimer model, shown as a solid line. The determined association constant was in the range 1–5 μM.
