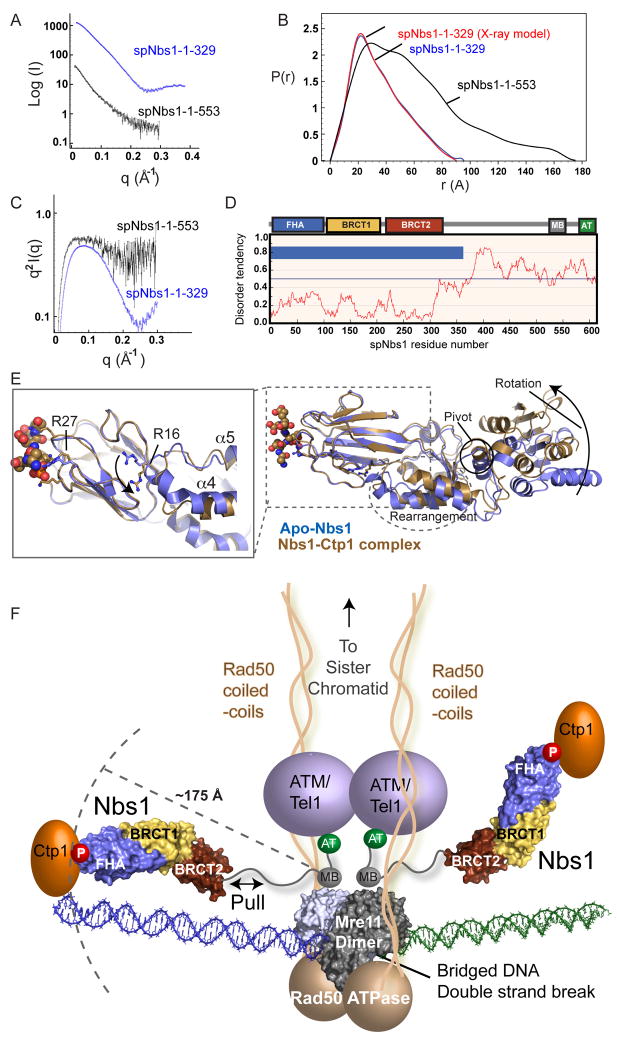
Figure 7. Nbs1 is an Extended, Flexible, Effector and Signaling Scaffold.
(A) spNbs1-fc (1–329) (blue) and spNbs1-ΔAT (1–553) (black) proteins were characterized by SAXS.
(B) SAXS electron pair distribution function shows spNbs1ΔAT is elongated in solution.
(C) SAXS Kratky plot indicates spNbs1ΔAT is only partially folded in solution.
(D) S. pombe Nbs1 order-disorder predictions from IUPRED. Predicted ordered (blue bars) and unstructured regions (no blue bars) shows stretches of disorder tendency > 0.5.
(E) Structural overlay of Ctp1-bound (brown) and apo-Nbs1 (blue) crystal forms shows BRCT1-BRCT2 interface rotation and helical rearrangements at the FHA-BRCT1 interface. Left inset: An FHA domain arginine switch (R16) toggles between two conformational states, directly linking the FHA to motions to the BRCT domains.
(F) Model of MRN-Ctp1 complex bound at a bridging DNA DSB. The flexible Nbs1 C-terminus links FHA-bound Ctp1 to an Mre11-Rad50 heterotetrameric core complex bridging a DNA double strand break.