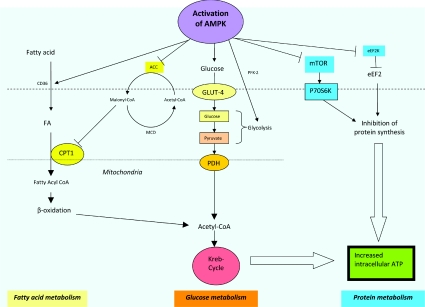
Figure 2. AMPK activation leads to activation of different metabolic pathways.
AMPK plays an important role in whole-body energy homoeostasis as it regulates and interacts with different key metabolic pathways. Activation of AMPK, secondary to a change in the AMP/ATP ratio or activation by upstream kinases, such as CAMKK (CaMK kinase) and LKB1 leads to a switching on of energy-production pathways, such as glucose and lipid metabolism, and a turning off of energy-metabolic processes, such as protein synthesis, which is not required for immediate cell survival. (i) Fatty acid metabolism. AMPK activation leads to increased translocation of CD36, a fatty-acid-transport protein, which increases fatty acid (FA) flux into cells and subsequent uptake into mitochondria for β-oxidation. CPT-1 inhibits fatty acid influx and acts as a gatekeeper for the mitochondrial uptake of fatty acids. Activation of AMPK leads to the inhibition of ACC2, which normally converts acetyl-CoA into malonyl-CoA. The inhibitory effect of malonyl-CoA on CPT-1 is hence removed, leading to unopposed intake of fatty acids into mitochondria. Furthermore, phosphorylating and inactivation of ACC1 by AMPK activation reduces fatty acid synthesis and turns off the expression of lipogenic genes, such as fatty acid synthase. MCD, malonyl-CoA decarboxylase. (ii) Glucose metabolism. Activation of AMPK increases translocation and retention of GLUT-4 in the plasma membrane, as well as increased transcription of the GLUT-4 gene, leading to increased glucose uptake. It also enhances glycolysis via activation and phosphorylation of PFK-2. (iii) Protein metabolism. p70RSK (p70S6K) is a one of the key kinases involved in protein synthesis. mTOR activates p70RSK and leads to increased protein synthesis. When AMPK is activated, the activation of p70RSK is blocked as a result of the inhibition of mTOR. Activation of AMPK also results in phosphorylation and inactivation of eEF2, and subsequent inhibition of protein synthesis. eEF2K, eEF2 kinase.