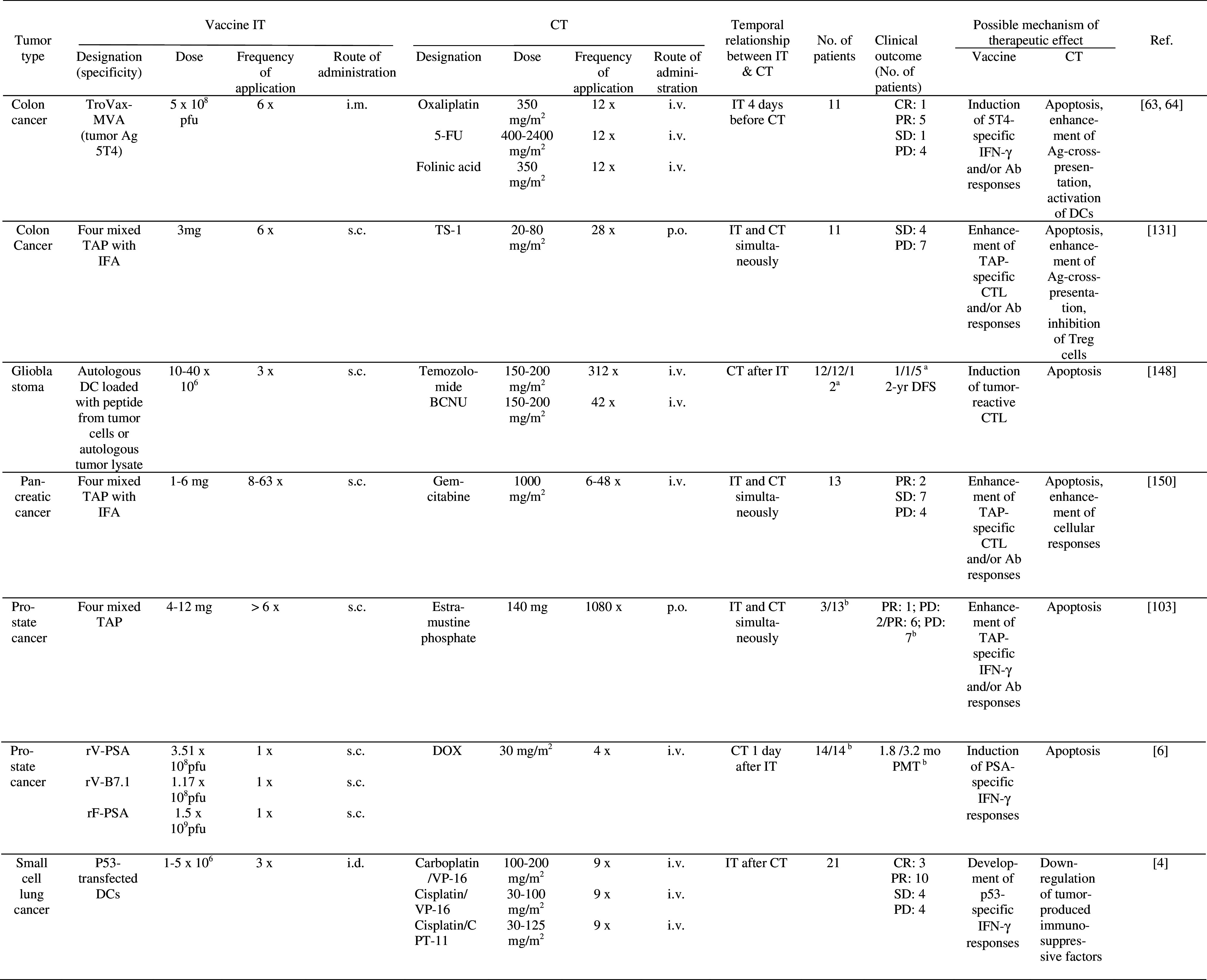
Table 4.
Clinical trials of combined active specific IT and CT
aCT/IT/CT + IT
bIT/IT + CT
Ab antibody, AML acute myelogenous leukemia, BCG Bacillus Calmette Guerin, BCNU 1, 3-bis-(2-chloroethyl)-1-nitrosourea, CPT-11 irinotecan, CR complete response, CT chemotherapy, CY cyclophosphamide, DC dendritic cells, DFS disease free survival, i.d. intradermally, IFA incomplete Freund’s adjuvant, IT immunotherapy, MR mixed response, MTX Methotrexate, MVA modified vaccinia Ankara, NR no response, NT not tested, OR objective (>50%) regression, PD progressive disease, p.o. per os, PMT progression median time, PR partial response, PSA prostate-specific antigen, rF recombinant fowlpox virus, rV recombinant vaccinia virus, s.c. subcutaneously, SD stable disease, TAP tumor associated peptides, TAX paclitaxel, TS-1 5-FU derivative, VP-16 etoposide
